融合工程CYP109E1和一个新的还原酶结构域用于25(OH)VD3生物合成
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
25-羟基维生素D3 (25(OH)VD3)是一种重要的日常营养补充剂,直接酶促C25羟基化维生素D3 (VD3)到25(OH)VD3是一种绿色和可持续的方法。然而,细胞色素P450的氧化还原伙伴的催化活性和电子转移效率较低,限制了其生产。在这里,CYP109E1的结构引导半遗传设计导致CYP109E1M2突变体,其25(OH)VD3的产量比野生型增加了2倍。此外,新的还原酶结构域BmRD首次被用于构建与CYP109E1M2的融合蛋白。值得注意的是,在BmRD的n端截断两个氨基酸产生融合蛋白Chimera-2,导致25(OH)VD3产量增加38.5%。在优化条件下,工程菌株03-2的25(OH)VD3产率为491.3 mg/L,转化率为49.0%,时空产率为61.4 mg/(L·h)。本研究证明了P450的修饰和优化潜力,为25(OH)VD3生物合成的产业化奠定了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
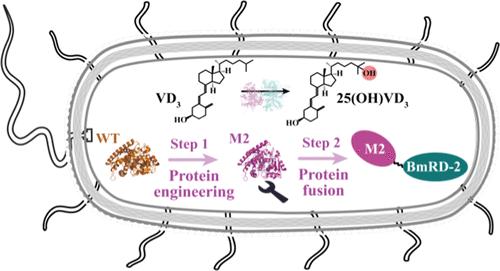
Fusing Engineered CYP109E1 and a New Reductase Domain for 25(OH)VD3 Biosynthesis
25-hydroxyvitamin D3 (25(OH)VD3) is an important daily nutritional supplement, and direct enzymatic C25 hydroxylation of vitamin D3 (VD3) to 25(OH)VD3 is a green and sustainable method. However, the low catalytic activity and electron transfer efficiency of redox partners of cytochrome P450 limited its production. Here, structure-guided semirational design of CYP109E1 led to the mutant CYP109E1M2, which showed a 2-fold increase in 25(OH)VD3 production compared to the wild-type. Furthermore, the novel reductase domain BmRD was first employed to construct a fusion protein with CYP109E1M2. Notably, truncating only two amino acids at the N-terminus of BmRD generated a fusion protein Chimera-2, resulting in a 38.5% increase in 25(OH)VD3 production. Under optimized conditions, the engineered strain Escherichia coli 03-2 produced 491.3 mg/L 25(OH)VD3 with a conversion of 49.0% and a space-time yield of 61.4 mg/(L·h). This work demonstrates the modification and optimization potential of P450 and lays a theoretical foundation for the industrialization of the 25(OH)VD3 biosynthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


