利用全细胞催化技术提高亮氨酸-亮氨酸二肽的产量
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
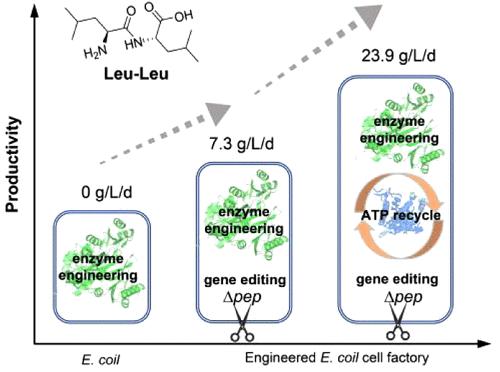
亮氨酸-亮氨酸二肽(Leu-Leu)因其促进肌肉生长、促进恢复和减少疲劳的能力而被公认,显示出治疗肌肉减少症和代谢紊乱等疾病的潜力。近年来,亮氨酸的生物合成研究日益受到关注;然而,它的生产受到催化效率低、肽键水解为副反应和副产物生成三肽的阻碍。本研究通过数据挖掘技术,从无硅假单胞菌中鉴定出一种新的l -氨基酸连接酶(Lal)。该连接酶能有效催化l-Leu转化为Leu-Leu,其kcat/Km值为14.6 s-1 M-1,熔化温度为57℃。此外,大肠杆菌中四种内源性肽酶的破坏通过全细胞催化导致亮氨酸-亮氨酸二肽的积累。腺苷5 ' -三磷酸(ATP)的再生是由来源于丹毒杆菌的多磷酸激酶的同时作用促进的。在优化条件下,共表达L-氨基酸连接酶和多磷酸激酶的大肠杆菌底盘在ATP自给自足的情况下实现了23.85 g/L/d的时空产率。我们的研究增强了Lals在二肽合成中的能力,并强调了它们在亮氨酸-亮氨酸二肽过剩生产中的潜在应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced Production of Leucine–Leucine Dipeptide by Whole-Cell Catalysis
The Leucine–Leucine dipeptide (Leu-Leu) is recognized for its ability to enhance muscle growth, promote recovery, and reduce fatigue, demonstrating therapeutic potential for conditions such as sarcopenia and metabolic disorders. Recent research in the biosynthesis of the Leu-Leu has gained increasing attention; however, its production is hampered by low catalytic efficiency, hydrolysis of the peptide bond as side reaction, and the generation of tripeptides as byproducts. In this study, a novel L-amino acid ligase (Lal) from Pseudomonas sessilinigenes was identified through data mining. This ligase effectively catalyzes the conversion of l-Leu to the Leu-Leu, exhibiting a kcat/Km value of 14.6 s–1 M–1 and a melting temperature of 57 °C. Furthermore, the disruption of four endogenous peptidases in Escherichia coli (E. coli) led to the accumulation of Leu-Leu dipeptide via whole-cell catalysis. The regeneration of adenosine 5′-triphosphate (ATP) is facilitated by the simultaneous action of a polyphosphate kinase derived from Erysipelotrichaceaebacterium. The engineered E. coli chassis, which coexpresses the L-amino acid ligase and polyphosphate kinase, achieves a space-time yield of 23.85 g/L/d in an ATP self-sufficient manner under optimized conditions. Our study enhances the capabilities of Lals in the synthesis of dipeptides and highlights their potential application for the overproduction of the Leu-Leu dipeptide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


