通过对映选择毒性动力学、分子对接和对映体转化分析评价芬布康唑单体的安全性
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
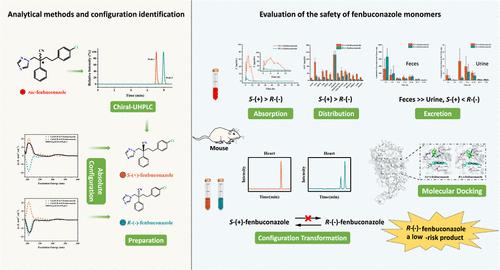
芬布康唑是一种手性三唑类杀菌剂,以消旋体的形式生产和使用。以往关于非目标生物中芬咯菌唑的毒理学研究主要使用外消旋体,因此有必要调查每种对映体的分布和消除情况,以便进行安全评估。在本研究中,首先通过 ECD 确认了芬布康唑对映体的绝对构型,并根据其光学活性将其指定为 S-(+)-fenbuconazole 和 R-(-)-fenbuconazole 对映体。选择超高效液相色谱-质谱/质谱法系统研究了芬布康唑对映体在小鼠体内的毒代动力学和对映体转化。结果表明,S-(+)-芬布康唑具有明显的对映体选择性,其AUC0-∞比R-(-)-芬布康唑高15.11倍,表明其血液吸收率更高。在涉及 14 个受检组织的分布实验中,除胃部外,S-(+)-芬布康唑的含量始终超过 R-(-)-芬布康唑。值得注意的是,肝脏中的 S-(+)-fenbuconazole 浓度仅次于胃,是 R-(-)-fenbuconazole 的 4.35 倍,这表明肝脏的蓄积倾向更大。分子对接研究进一步表明,S-(+)-芬布康唑与肝脏中的 CYP2B 酶之间的相互作用更强,这意味着其肝脏毒性潜力更大。这两种对映体很少从尿液或粪便中排出,累积排泄率低于 2.5‰。小鼠体内的对映体转换是单向的(R → S),在大多数组织中的转换率普遍较低。因此,对映体转换并不是驱动对映体选择性的主要因素。总之,R-(-)-芬布康唑的吸收性差,分布有限,与 CYP2B 酶的相互作用较弱,可被视为一种低风险产品,可指导单体开发并促进减少杀虫剂的使用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation of the Safety of Fenbuconazole Monomers via Enantioselective Toxicokinetics, Molecular Docking and Enantiomer Conversion Analyses
Fenbuconazole, a chiral triazole fungicide, is produced and used as a racemate. Previous toxicological research on fenbuconazole in nontarget organisms primarily used the racemate, necessitating an investigation into each enantiomer’s distribution and elimination for safety assessment. In this study, the absolute configurations of fenbuconazole enantiomers were first confirmed by ECD, designating them as S-(+)-fenbuconazole and R-(−)-fenbuconazole based on their optical activity. The UHPLC-QQQ/MS method was selected to systematically study the toxicokinetics and enantiomer conversion of fenbuconazole enantiomers in mice. The results revealed significant enantioselectivity, with S-(+)-fenbuconazole exhibiting 15.11 times higher AUC0–∞ than R-(−)-fenbuconazole, indicating greater blood absorption. In the distribution experiment involving the 14 examined tissues, S-(+)-fenbuconazole consistently exceeded R-(−)-fenbuconazole levels, except in the stomach. Notably, S-(+)-fenbuconazole concentration in the liver was second only to the stomach and was 4.35 times higher than R-(−)-fenbuconazole, suggesting a greater propensity for hepatic accumulation. Molecular docking studies further demonstrated a stronger interaction between S-(+)-fenbuconazole and the CYP2B enzyme in the liver, implying higher hepatotoxic potential. Both enantiomers were rarely excreted in urine or feces, with a cumulative excretion rate below 2.5‰. Enantiomer conversion occurred unidirectionally (R → S) in mice, and the rates were generally low in most tissue. Thus, enantiomeric conversion was not the primary factor driving the enantioselectivity. In summary, R-(−)-fenbuconazole exhibited poor absorption, limited distribution, and a weak interaction with the CYP2B enzyme, which may be considered a low-risk product that could guide monomer development and promote the reduction of pesticide usage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


