用于监测定量黏度-细胞迁移关系的超灵敏定量迁移传感器
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
细胞外黏度与细胞迁移的关系是提示肿瘤生长转移的重要新线索。然而,它们的数量关系尚未被揭示。本研究首次研制了一种超灵敏定量迁移传感器(UQMS),可定量监测细胞外粘度异常变化及异常情况下细胞迁移率,检测限破纪录地达到3个细胞。在这个UQMS中,一个强大的葡萄糖/O2燃料电池(GFC)可以在体液中稳定工作,并可以输出连续的电信号,作为能量驱动器和信号发生器。在GFC的阳极,我们设计了一个距离电活性区域2毫米的细胞生长区域,以确保电活性区域最初不受细胞干扰。细胞外粘度的升高阻碍了传质,导致GFC电流输出的瞬时线性下降。随着时间的推移,癌细胞迁移到阳极上的电活性区域,这进一步阻碍了电子和质量的传递,导致GFC的电流输出随时间和细胞数量的减少。通过分析GFC电流输出在不同时间段内的变化,UQMS可以定量检测细胞外黏度,范围为1 cP - 27 cP,可以区分正常和异常黏度;此外,可以在低至3个细胞的水平上建立长期贴壁细胞迁移与粘度之间的定量关系。该UQMS可定量监测不同黏度下贴壁细胞(ATCs)和循环肿瘤细胞(CTCs)的迁移情况。我们观察到,高粘度使ATC能够以节能模式快速变形迁移,但减缓了CTC的迁移;ctc的迁移速度明显快于ATCs。这项工作有望对评估ATCs和CTCs迁移引起的肿瘤转移风险有很大帮助。本文章由计算机程序翻译,如有差异,请以英文原文为准。
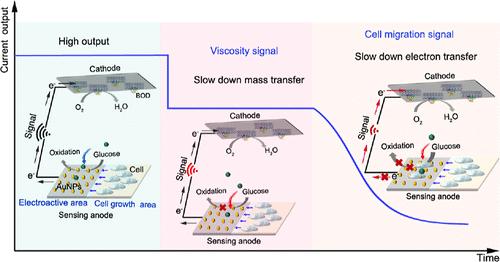
Ultrasensitive Quantitative Migration Sensor for Monitoring the Quantitative Viscosity–Cell Migration Relationship
The relationship between extracellular viscosity and the cells’ migration is a new and crucial clue indicating tumor growth and metastasis. However, their quantitative relationship has not yet been revealed. In this study, an ultrasensitive quantitative migration sensor (UQMS) that can quantitatively monitor the abnormal change of viscosities and the cell migration rate under abnormal extracellular viscosities with a record-breaking detection limit of 3 cells is developed for the first time. In this UQMS, a robust glucose/O2 fuel cell (GFC) that can work steadily in body fluids and can output a continuous electrical signal serves as the energy driver and signal generator. At the anode of the GFC, we design a cell growth area two millimeters away from the electroactive area to ensure that the electroactive area is initially free from cell interference. The raised extracellular viscosity impedes mass transfer, leading to an instantaneous and linear decrease in the current output of the GFC. With the time going, the cancer cells migrate to the electroactive area on the anode, which further blocks the electron and mass transfer, leading to a time- and cell-number-dependent decrease in the current output of the GFC. By analyzing changes of the GFC’s current output during different timeframes, the UQMS can quantitatively detect the extracellular viscosity in a wide range (1 cP–27 cP) that could distinguish the normal and abnormal viscosity; moreover, the quantitative relationship between long-term adherent cell migration and viscosities can be built at a level as low as 3 cells. Both of the migrations of adherent cells (ATCs) and circulating tumor cells (CTCs) under different viscosities can be quantitatively monitored by this UQMS. And we observe that the high viscosity enables the ATC to deform to migrate rapidly in an energy-efficient mode but slows down CTC migration; what is more, the migration of CTCs is significantly faster than that of ATCs. This work is expected to be highly helpful in assessing the risk of tumor metastasis from the migration of both ATCs and CTCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


