设计羰基还原酶高效合成光纯(S)-原木聚糖:一种替代合成途径
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
原木聚糖是化妆品中具有生物活性的c -糖苷化合物。传统的方法是将山毛榉树中的d-木糖转化为1-C-(β-d-木吡喃基)丙酮,并用硼氢化钠还原。这种方法产生硼酸盐作为副产品,需要一个繁琐的去除过程。与(R)-Pro-Xylane相比,(S)-Pro-Xylane具有更强的生物活性。因此,本研究将重点放在还原反应的第二步,从念珠菌正硅氧体Co 90-125 (CoCR13)中设计羰基还原酶(CR)。变体R129E/D210F的kcat/Km比野生型(WT)提高了245.33倍,并有>;99% (β, S)非对异构体过剩。该反应在1300 mM底物下的时空产率为49.97 g·L-1·h-1。分子动力学模拟表明,R129和D210的突变增加了生产底物结合状态的比例,导致隧道更短、更宽。本研究扩大了CR在(S)- pro -木聚糖替代和可持续生产中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
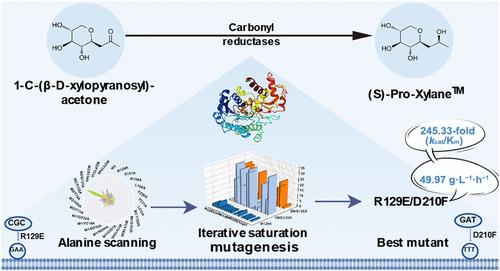
Engineering a Carbonyl Reductase for High-Efficiency Synthesis of Optically Pure (S)-Pro-Xylane: An Alternative Synthetic Route
Pro-Xylane is a biologically active C-glycoside compound in cosmetic products. The conventional method involves converting d-xylose from beech trees to 1-C-(β-d-xylopyranosyl)-acetone and reducing it with sodium borohydride. This method produced borate salts as byproducts, requiring a tedious removal process. In addition, (S)-Pro-Xylane shows superior biological activity compared to (R)-Pro-Xylane. Hence, with focus on the second step of the reduction reaction, the carbonyl reductase (CR) from Candida orthopsilosis Co 90–125 (CoCR13) was engineered in this study. Variant R129E/D210F showed a 245.33-fold improvement in kcat/Km over the wild-type (WT) with >99% (β, S) diastereomeric excess. The reaction achieved a spatiotemporal yield of 49.97 g·L–1·h–1 with a 1300 mM substrate. Molecular dynamics simulations suggest that mutations on R129 and D210 increased the proportion of productive substrate binding states and resulted in a shorter and wider tunnel. This study expands the utilization of CR for the alternative and sustainable production of (S)-Pro-Xylane.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


