基于共振能量转移的电化学发光传感器检测食品中盐酸氯四环素
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
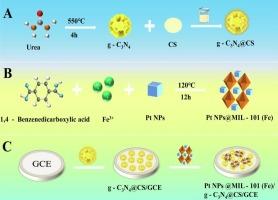
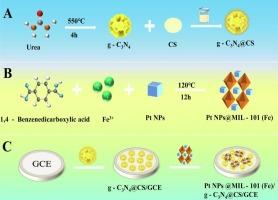
本研究构建了一种检测盐酸氯四环素(CTC)的高灵敏度电化学发光(ECL)传感器。将g- c3n4 -壳聚糖(g-C3N4@CS)和Pt NPs@MIL-101(Fe)的配合物修饰为电极。在g-C3N4- s2o82−体系中,g-C3N4作为ECL信号。合成的Pt NPs@MIL-101(Fe)作为该体系的新型助反应促进剂,进一步增强了ECL信号。随着CTC的加入,ECL信号减弱,这种猝灭现象主要是由于ECL- ret引起的,并对其机理进行了研究。在最佳条件下,ECL信号(ΔI)的变化与CTC浓度(lgCCTC)的对数(5 × 10−14 ~ 5 × 10−9 mol L−1)之间存在较强的线性关系。线性方程为ΔI = 16,817 + 1179.4 lgCCTC,相关系数为0.995。该方法检出限极低,为1.7 × 10−14 mol L−1 (S/N = 3),适用于实际样品分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrochemiluminescence sensor based on resonance energy transfer for detection of chlortetracycline hydrochloride in food
In this work, a highly sensitive electrochemiluminescence (ECL) sensor for Chlortetracycline hydrochloride (CTC) detection was constructed. The complex of g-C3N4-chitosan (g-C3N4@CS) and Pt NPs@MIL-101(Fe) were modified to the electrode. In g-C3N4-S2O82− system, g-C3N4 served as an ECL signal. Synthesized Pt NPs@MIL-101(Fe) acted as a novel coreaction promoter of the system, further enhancing the ECL signal. With the addition of CTC, the ECL signal decreased, and this quenching phenomenon was mainly because of ECL-RET and the related mechanism was studied. Under the best conditions, a strong linear relationship was observed between the change in ECL signal (ΔI) and the logarithm of CTC concentration (lgCCTC), spanning from 5 × 10−14 to 5 × 10−9 mol L−1. The linear equation was ΔI = 16,817 + 1179.4 lgCCTC with a correlation coefficient of 0.995. The method demonstrates an exceptionally low detection limit of 1.7 × 10−14 mol L−1 (S/N = 3), making it suitable for real sample analysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


