金属离子(Ca2+, Cu2+和Mg2+)与四肽FFDR的结合机制:实验和量子化学相结合的方法
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
先前的研究表明,从薏苡仁中提取的四肽FFDR具有抗氧化特性。在继续研究中,密度泛函理论(DFT)被用于研究FFDR与Ca2+, Cu2+和Mg2+的分子水平络合行为。用光谱学和细胞模型验证了这一点。电子性质表明,Mg-FFDR具有较低的能隙(1.733 eV),比Ca-FFDR具有更高的反应活性。分子中原子量子理论(QTAIM)显示所有金属氧键的拉普拉斯值均为正,表明存在具有闭壳相互作用特征的配位键。1H NMR结果显示j偶联模式与金属配位一致,而FTIR光谱显示参与金属结合的官能团的振动频率有明显变化。Mg-FFDR和Ca-FFDR均表现出明显的ROS清除活性,并增强HepG2细胞的SOD和CAT活性。这些发现为合理设计金属肽复合物作为功能性食品或营养保健品提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。

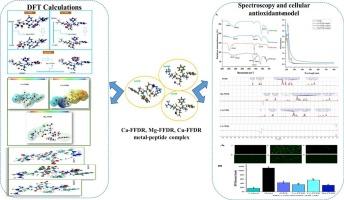
Binding mechanism of metal ions (Ca2+, Cu2+ and Mg2+) with tetrapeptide FFDR: A combined experimental and quantum chemistry approach
A previous study demonstrated that a tetrapeptide FFDR derived from coix seed possesses antioxidant properties. In continuation of the study, Density Functional Theory (DFT) was employed to investigate the molecular-level complexation behaviour of FFDR with Ca2+, Cu2+, and Mg2+. DFT predictions were validated using spectroscopy and cellular model. The electronic properties revealed that Mg-FFDR, with its lower energy gap (1.733 eV), exhibits higher reactivity compared to Ca-FFDR which displayed higher stability (8.180 eV). The Quantum Theory of Atoms in Molecules (QTAIM) showed positive Laplacian values for all metal‑oxygen bonds, indicating the presence of coordination bonds characteristic of closed-shell interactions. Results from 1H NMR spectra revealed J-coupling patterns consistent with metal coordination for Mg and Ca-peptide complexes. FTIR spectra displayed distinct changes in the vibrational frequencies of functional groups involved in metal binding for all complexes. Both Mg-FFDR and Ca-FFDR demonstrated significant ROS scavenging activities, and enhanced SOD and CAT activities in HepG2 cells. These findings serve as a baseline for the rational design of metal-peptide complexes as functional foods or nutraceuticals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


