细菌进化和氧适应的地质时间尺度
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
微生物生命在地球历史上占据主导地位,但留下的化石记录很少,这极大地阻碍了我们对深时间进化的理解。然而,细菌代谢在地球化学记录中留下了印记,最显著的是大氧化事件(GOE)。我们结合机器学习和系统发育调节来推断祖先细菌向有氧生活方式的转变,并将它们与GOE联系起来,以校准细菌时间树。现存细菌门的多样性可追溯到太古宙和元古代,以及显生宙之前的细菌科。我们推断,大多数细菌门的祖先是厌氧的,在GOE之后采用了好氧的生活方式。然而,在蓝藻祖先中,有氧代谢可能早于GOE,这可能促进了含氧光合作用的进化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
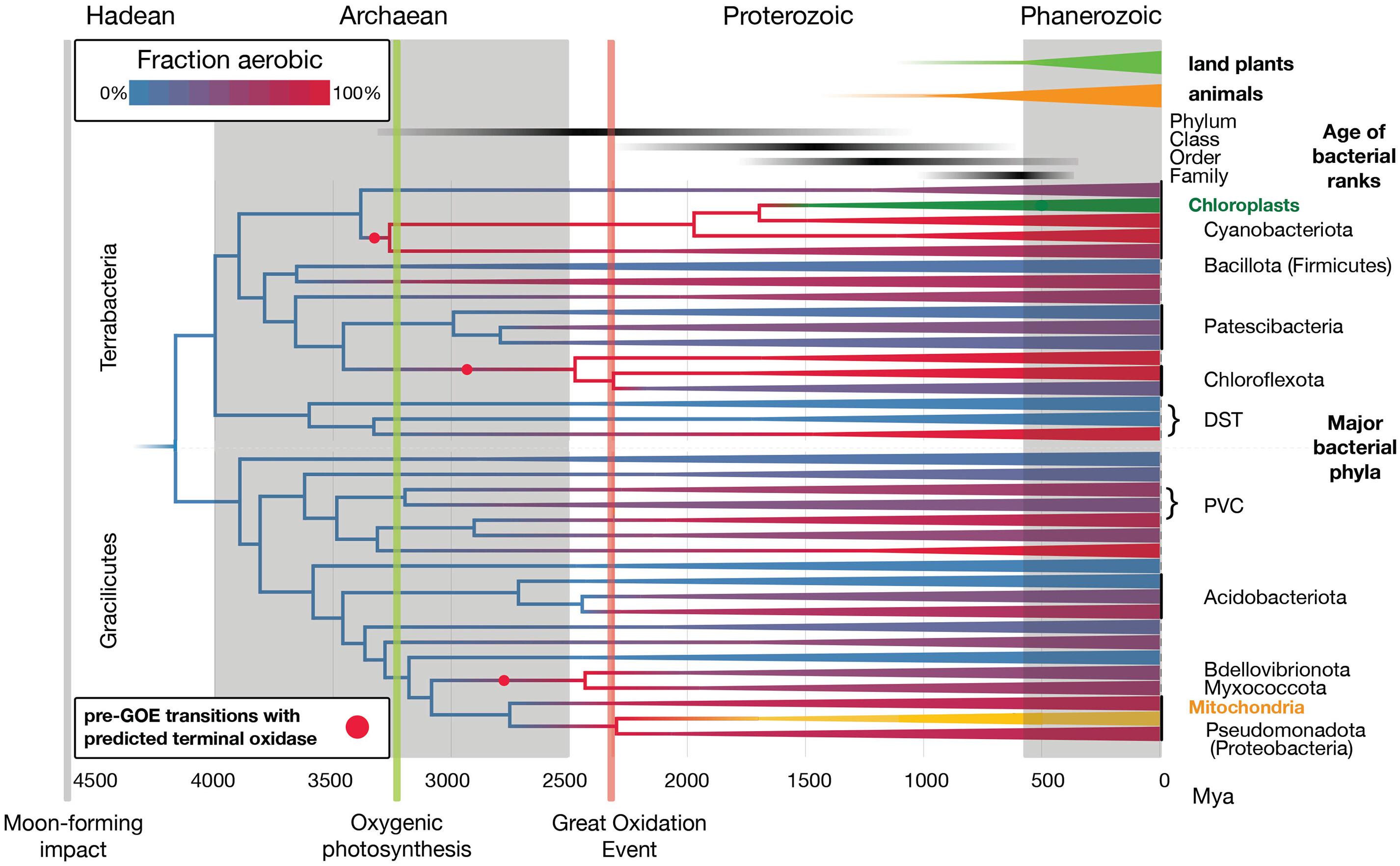
A geological timescale for bacterial evolution and oxygen adaptation
Microbial life has dominated Earth’s history but left a sparse fossil record, greatly hindering our understanding of evolution in deep time. However, bacterial metabolism has left signatures in the geochemical record, most conspicuously the Great Oxidation Event (GOE). We combine machine learning and phylogenetic reconciliation to infer ancestral bacterial transitions to aerobic lifestyles, linking them to the GOE to calibrate the bacterial time tree. Extant bacterial phyla trace their diversity to the Archaean and Proterozoic, and bacterial families prior to the Phanerozoic. We infer that most bacterial phyla were ancestrally anaerobic and adopted aerobic lifestyles after the GOE. However, in the cyanobacterial ancestor, aerobic metabolism likely predated the GOE, which may have facilitated the evolution of oxygenic photosynthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


