CRISPR/Cas12a 蛋白开关驱动的无标签电化学生物传感器用于灵敏检测病毒蛋白酶
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
病毒蛋白酶是病毒发病机制的关键分子靶点,是了解病毒感染机制和开发抗病毒治疗方法的关键生物标志物。本研究介绍了一种无标记的电化学生物传感器,该传感器通过将蛋白酶响应CRISPR/Cas蛋白开关(casps)与血红蛋白适配体功能化的电化学界面集成,实现了敏感的病毒蛋白酶检测。该生物传感器的机制依赖于病毒蛋白酶介导的蛋白水解,这导致CasPSs释放活性Cas12a蛋白,并通过连续切割固定化氧化还原活性血红蛋白/适体复合物产生放大的电化学反应。该生物传感器具有飞摩尔灵敏度,实现了对丙型肝炎病毒NS3/4A蛋白酶的特异性检测,并且通过替换CasPS模块,可以很容易地扩展到其他病毒蛋白酶。通过监测不同病毒载量和感染后时间的肠道病毒71 3C蛋白酶活性,验证了该生物传感器的可行性。该研究为CRISPR生物传感与电化学平台的整合提供了一种有前景的策略,为病毒感染监测和抗病毒药物筛选提供了有用的分析工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
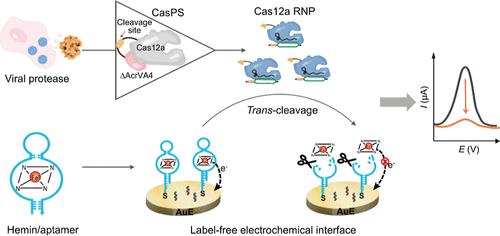
CRISPR/Cas12a Protein Switch Powered Label-Free Electrochemical Biosensor for Sensitive Viral Protease Detection
Viral proteases are critical molecular targets in viral pathogenesis, representing pivotal biomarkers for understanding viral infection mechanisms and developing antiviral therapeutics. This study introduces a label-free electrochemical biosensor that enables sensitive viral protease detection by integrating protease-responsive CRISPR/Cas protein switches (CasPSs) with a hemin aptamer-functionalized electrochemical interface. The biosensor’s mechanism relies on viral protease-mediated proteolysis, which leads to the release of active Cas12a proteins from CasPSs and generates amplified electrochemical responses through continuous cleavage of immobilized redox-active hemin/aptamer complexes. This biosensor achieved specific hepatitis C virus NS3/4A protease sensing with femtomolar sensitivity and could be readily expanded to other viral proteases by replacing the CasPS module. The feasibility of this biosensor was demonstrated by monitoring enterovirus 71 3C protease activities in virus-infected cell samples with different viral loads and postinfection times. This study provides a promising strategy for integrating CRISPR biosensing with electrochemical platforms, offering a helpful analytical tool for viral infection monitoring and antiviral drug screening.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


