一株葡酸柠檬酸杆菌的诱变及产透明质酸酶特性研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
透明质酸酶是一种糖苷酶,具有降解透明质酸的能力。它在医疗和化妆品领域内使用,并且代表了制备透明质酸低聚糖的有效方法。透明质酸酶活性的增加已被证明是促进低分子量透明质酸生产的有效方法。本研究从自然室温保存的透明质酸钠溶液中鉴定出具有自主知识产权的透明质酸活性降解菌株葡质酸柠檬酸杆菌(Citrobacter portucalensis HA23)。为了提高菌株的产酶能力,我们采用常压和室温等离子体(ARTP)技术选育了产酶能力增强的突变体HA2301。突变菌株HA2301的酶活性比HA23提高了58.82%。在以酵母粉为碳源、花生饼粉为氮源的培养基中,以pH 5.5、37℃、250 rpm为最佳培养条件,菌株酶活性达到25808 U mL-1。对酶与透明质酸钠的反应溶液进行了表征,主要产物为HA2和HA4。本文为其工业应用提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
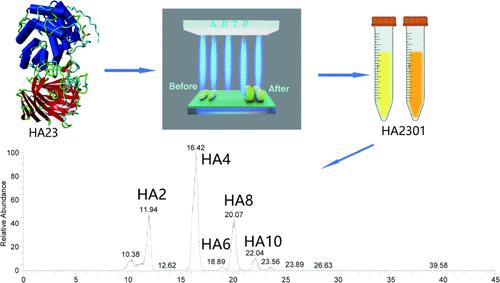
Mutagenesis and Characterization of Hyaluronidase Production by a New Isolate of Citrobacter portucalensis
Hyaluronidase, a glycosidase, has the capacity to degrade hyaluronic acid. It is employed within both the medical and cosmetic domains and represents an efficacious methodology for the preparation of hyaluronic acid oligosaccharides. The increase in the activity of hyaluronidase has been demonstrated to be an effective method of promoting the production of low molecular weight hyaluronic acid. In the present study, we identified the active hyaluronan-degrading strain Citrobacter portucalensis HA23 from a sodium hyaluronate solution kept at natural room temperature, with independent intellectual property rights. To enhance the strain’s enzyme-producing capacity, we employed atmospheric and room temperature plasma (ARTP) technology to select and breed the mutant HA2301 with augmented enzyme-producing capabilities. The enzyme activity of the mutant strain HA2301 was observed to have increased by 58.82% in comparison to that of HA23. It was established that pH 5.5, 37 °C, and 250 rpm represented the optimal culture conditions, with the enzyme activity of the strain reaching 25808 U mL–1 in a medium comprising a carbon source of yeast flour and a nitrogen source of peanut cake flour. The reaction solution of the enzyme with sodium hyaluronate was characterized, with the main products identified as HA2 and HA4. The present text provides a reference for its industrial application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


