不饱和脂肪酸甲酯的温度依赖性动力学:模拟自氧化机制
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
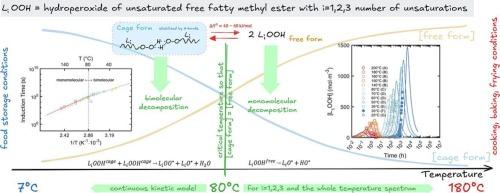
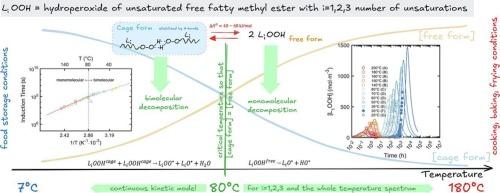
了解不饱和脂肪酸甲酯(FAMEs)的氧化稳定性对食品保鲜和工业应用至关重要。该研究扩展了我们的第一性原理动力学模型(Food Res Int 2023, 173, 113289),将机理见解整合到从双分子到单分子氢过氧化氢分解的温度依赖转变中。我们的方法基于临界温度的实验证据和自由氢过氧化物和氢键氢过氧化物之间平衡的参数化,在7 °C到200 °C(391次测量)的数据中得到了验证。未经进一步拟合,模型得到的R2值在0.613 ~ 0.896之间。在整理的数据集上,一致性相关系数超过0.78,预测区间覆盖概率超过50 %,相对偏差误差低于30 %。在80 °C以下,双分子途径占主导地位,而在较高温度下,单分子过程占主导地位,影响诱导时间和氧化速率。这些发现为通过整合FAME成分和温度历史来优化保质期的预测模型奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Temperature-dependent kinetics of unsaturated fatty acid methyl esters: Modeling autoxidation mechanisms
Understanding the oxidative stability of unsaturated fatty acid methyl esters (FAMEs) is critical for food preservation and industrial applications. This study extends our first-principles kinetic model (Food Res Int 2023, 173, 113289) by integrating mechanistic insights into the temperature-dependent shift from bimolecular to monomolecular hydroperoxide decomposition. Our approach, based on experimental evidence of a critical temperature and the parameterization of the equilibrium between free and hydrogen-bonded hydroperoxides, was validated against data spanning 7 °C to 200 °C (391 measurements). Without further fitting, the model achieves R2 values between 0.613 and 0.896. On curated datasets, the Concordance Correlation Coefficient exceeds 0.78, the Prediction Interval Coverage Probability is over 50 %, and relative deviation errors below 30 %. Below 80 °C, bimolecular pathways dominate, whereas monomolecular processes prevail at higher temperatures, influencing induction times and oxidation rates. These findings lay the groundwork for predictive models that optimize shelf life by integrating FAME composition and temperature history.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


