通过非催化袋和催化袋同步工程提高l-鼠李糖异构酶对d-近醛合成的催化效率
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
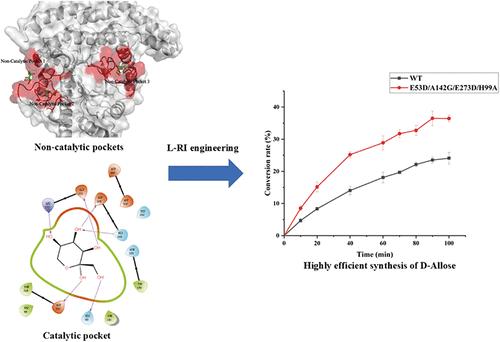
d-Allose是一种重要的稀有糖,在食品、制药和医疗保健行业具有重要的应用价值。l-鼠李糖异构酶(l-RI)催化d-allulose转化d-allulose是生产d- allolose的最常用方法。然而,该酶对d-allulose的催化效率相对较低。为了提高粪状梭菌衍生的l-RI的催化效率,对非催化口袋中的关键氨基酸进行了突变,以提高d-allulose进入催化口袋的概率,并增加活性区域底物的局部浓度。同时,通过突变氨基酸His99,降低了空间位阻对催化袋盖的影响。值得注意的是,组合突变体E53D/A142G/E273D/H99A对d-allulose的催化效率比野生型酶提高了170%。此外,表达该L - ri变体的枯草芽孢杆菌(Bacillus subtilis) 168个全细胞在90分钟内从100 g/L的d-allulose转化为d-allose,转化率为36.5%。该研究显示了一种具有工业生产d-allose潜力的高效生物催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing the Catalytic Efficiency of l-Rhamnose Isomerase for d-Allose Synthesis through Simultaneous Engineering of Noncatalytic and Catalytic Pockets
d-Allose is a crucial rare sugar that holds significant application value in the food, pharmaceutical, and healthcare industries. The most prevalent method for the production of d-allose is its conversion from d-allulose, which is catalyzed by l-rhamnose isomerase (l-RI). However, this enzyme demonstrates relatively low catalytic efficiency toward d-allulose. To enhance the catalytic efficiency of l-RI derived from Clostridium stercorarium, key amino acids in the noncatalytic pockets were mutated to improve the probability of d-allulose entering the catalytic pocket and to increase the local concentration of the substrate in the active region. Simultaneously, the impact of steric hindrance on the lid around the catalytic pocket was reduced by mutating the amino acid His99. Notably, the catalytic efficiency of the combined mutant E53D/A142G/E273D/H99A toward d-allulose was increased by 170% compared to that of the wild-type enzyme. Moreover, Bacillus subtilis 168 whole cells expressing this l-RI variant achieved a 36.5% conversion rate of d-allose from 100 g/L d-allulose within 90 min. This study presents a highly efficient biocatalyst with the potential for industrial production of d-allose.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


