具有多酶活性的 S 修饰 MOF 纳米酶级联系统用于双模式抗生素检测
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
抗生素的合理使用为人类健康建立了坚固的堡垒。然而,它们的不当使用对环境造成了有害的后果,强调了开发有效和具有成本效益的检测和降解平台的必要性。本文研究了一种具有非铜活性中心的硫修饰铁钴双金属单原子氮掺杂碳催化剂(S-FeCo-NC)。与以铜为活性中心的传统漆酶不同,S-FeCo-NC催化剂具有多种酶活性,包括类漆酶、类过氧化物酶和类过氧化氢酶的功能,其中铁和钴作为活性中心。作为概念验证,将S-FeCo-NC的漆酶样和过氧化氢酶样联合功能作为独立的信号输出,同时设计了多酶级联双模式检测系统,用于与过氧化物酶样酶联合快速检测四环素(TC)。在该体系中,氧作为电子受体直接参与了类漆酶的催化过程,而类过氧化氢酶的过氧化物酶则有效催化H2O2生成O2。O2浓度的升高为漆酶样酶的催化活性增加提供了独特的优势,该酶使用稳定的4-氨基吡啶氧化TC输出视觉分辨的比色信号。此外,S-FeCo-NC具有过氧化物酶样活性,催化生成具有强氧化性的OH自由基,这些自由基对TC进行有效的氧化分解。采用差分脉冲循环伏安法对催化过程的响应信号输出进行分析,进一步提高了检测的灵敏度和准确性。实验结果表明,该检测系统在0.005 ~ 500 μM范围内对TC具有良好的响应信号,检测范围分别达到0.5 ~ 500 μM和0.005 ~ 1.00 μM,检测限分别低至0.22 μM和1.68 nM。这种基于多酶活性的级联双模检测系统已被证明可以显著提高漆酶的催化活性,同时在较低的检测范围内也表现出稳定性。这表明它可能为环境污染物的敏感检测和降解提供一种新的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
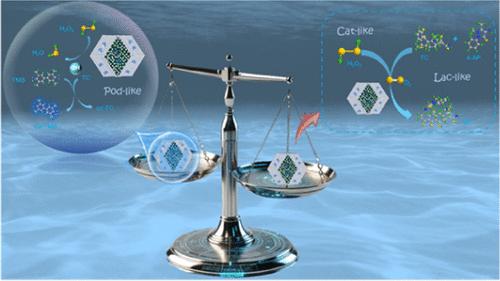
S-Modified MOF Nanozyme Cascade System with Multi-Enzyme Activity for Dual-Mode Antibiotic Assay
The judicious utilization of antibiotics has established a robust bulwark for human health. However, their improper usage has engendered deleterious ramifications on the environment, underscoring the imperative for developing efficacious and cost-effective detection and degradation platforms. This study presents a sulfur-modified iron–cobalt bimetallic single-atom nitrogen-doped carbon catalyst (S-FeCo-NC) with a noncopper active center. In contrast to conventional laccase, which utilizes copper as its active center, the S-FeCo-NC catalyst exhibits multiple enzyme activities, including laccase-like, peroxidase-like, and catalase-like functions, with iron and cobalt serving as the active centers. As a proof of concept, the combined laccase-like and catalase-like functions of S-FeCo-NC were used as independent signal outputs, while a multienzyme cascade dual-mode assay system was designed for the rapid detection of tetracycline (TC) in combination with peroxidase-like enzymes. In this system, oxygen directly participated in the catalytic process of laccase-like as an electron acceptor, while catalase-like peroxidase efficiently catalyzed the production of O2 from H2O2. The elevated concentration of O2 offered a unique advantage for the increased catalytic activity of the laccase-like enzyme, which outputs visually resolved colorimetric signals using stable 4-aminopyridine with oxidized TC. Furthermore, the peroxidase-like activity of S-FeCo-NC catalyzed the generation of OH radicals with strong oxidative properties, and these radicals carried out effective oxidative decomposition of TC. The signal output of the response of the catalytic process was performed using differential pulse cyclic voltammetry, which further improved the sensitivity and accuracy of the detection. The experimental findings demonstrate that the detection system exhibits a favorable response signal to TC within the range of 0.005–500 μM, with its detection range reaching 0.5–500 and 0.005–1.00 μM, respectively, and the detection limit is as low as 0.22 μM and 1.68 nM, respectively. This cascade dual-mode detection system, based on multienzyme activity, has been shown to significantly enhance the catalytic activity of laccase, while also demonstrating stability in a lower detection range. This suggests that it may offer a novel approach for the sensitive detection and degradation of environmental pollutants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


