反式茴香醚代谢激活在小鼠肝细胞毒性中的作用
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
反式茴香醚(1-甲氧基-4-[1(E)-丙烯]苯,tAT)是八角茴香果实精油的主要成分。tAT侧链中的双键是一种警戒结构,可被代谢成环氧化物,可能导致肝损伤。这项工作调查并鉴定了tAT的反应性代谢物,这些代谢物与生物硫醇有化学反应,如谷胱甘肽(GSH)、n -乙酰-l-半胱氨酸(NAC)和蛋白质的半胱氨酸残基。在胆汁和尿液中分别发现了四种GSH偶联物(M2-M5)和两种NAC偶联物(M6和M7),它们分别来源于tAT的环氧化物代谢物。四种半胱氨酸加合物(M8-M11)在给药小鼠的蛋白水解肝蛋白中被发现。蛋白质内聚的形成表现出剂量依赖性。细胞色素P450 1A (CYP1A)主导了tAT的侧链环氧化。暴露于tAT诱导培养小鼠原代肝细胞的细胞毒性。α-萘黄酮预处理肝细胞可降低肝细胞对tAT细胞毒性的敏感性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
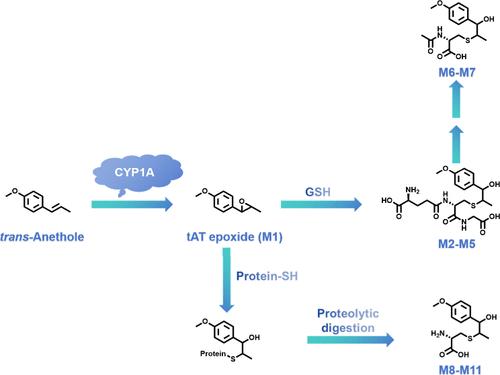
Metabolic Activation of trans-Anethole Plays a Role in Hepatic Cytotoxicity in Mice
trans-Anethole (1-methoxy-4-[1(E)-propenyl]benzene, tAT) is the main ingredient in the essential oil extracted from star anise fruits. The double bonds in the side chain of tAT are a type of alert structure that can be metabolized into epoxides possibly causing liver damage. This work investigated and identified the reactive metabolites of tAT that are chemically reactive to biothiols, such glutathione (GSH), N-acetyl-l-cysteine (NAC), and cysteine residues of proteins. Four GSH conjugates (M2–M5) and two NAC conjugates (M6 and M7), which are derived from epoxide metabolite of tAT, were found in bile and urine, respectively. Four cysteine adducts (M8–M11) were found in proteolytic liver proteins isolated from mice administered tAT. The formation of protein adduction exhibited a dose-dependent pattern. Cytochrome P450 1A (CYP1A) dominated the side chain epoxidation of tAT. Exposure to tAT induced cytotoxicity in cultured mouse primary hepatocytes. Pretreatment of hepatocytes with α-naphthoflavone decreased the susceptibility of the cells to tAT cytotoxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


