重组人XVII型胶原蛋白片段的发现及功能表征
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
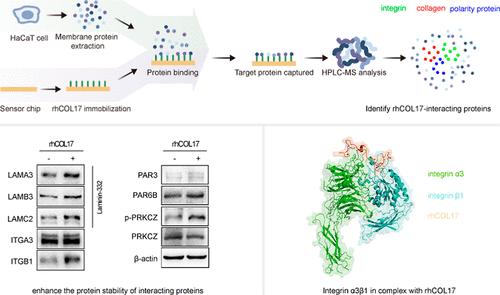
COL17A1主要表达于皮肤上皮细胞,主要定位于半粒内。它在表皮真皮附着中起着重要作用。因此,具有低分子量和高生物相容性的重组人样COL17A1蛋白(rhCOL17)是一种有前景和竞争力的生物材料。rhCOL17主要由氨基酸残基Gly659-Leu720组成,本研究旨在进一步了解rhCOL17的生物学功能和潜在的分子机制。利用表面等离子体共振(SPR)和液相色谱-串联质谱(LC-MS /MS)相结合的方法,我们鉴定了HaCaT细胞中rhCOL17的相互作用伴侣蛋白。其中包括几种胶原蛋白、整合素和细胞极性蛋白。rhCOL17处理后,laminin-332、整合素β1、细胞极性蛋白PAR-3、PAR-6B的表达水平上调,PRKCZ、AKT、TGF-β1信号通路被激活。此外,rhCOL17被发现促进细胞增殖并减轻紫外线辐射引起的损伤,部分是通过调节这些相互作用的蛋白质及其相关的信号通路。利用AlphaFold2和分子动力学模拟分析表明,rhCOL17肽稳定而紧密地结合在整合素α3和β1亚基之间的典型配体结合位点上。这些发现突出了rhCOL17在抗衰老领域的潜在多功能性和应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery and Functional Characterization of a Recombinant Fragment of Human Collagen Type XVII
COL17A1 is predominantly expressed in skin epithelial cells and primarily localized within hemidesmosomes. It plays an essential role in epidermal–dermal attachment. Consequently, a recombinant human-like COL17A1 protein (rhCOL17) with low molecular weight and high biocompatibility presents a promising and competitive biomaterial. The aim of this study is to gain more insight into the biological functions and underlying molecular mechanisms of rhCOL17, which primarily consists of amino acid residues Gly659-Leu720. Using a combination of surface plasmon resonance (SPR) and liquid chromatography-tandem mass spectrometry (LC–MS/MS), we identified the interacting partner proteins of rhCOL17 in HaCaT cells. These included several collagens, integrins, and cell polarity proteins. Upon rhCOL17 treatment, the expression levels of laminin-332, integrin β1, and the cell polarity proteins PAR-3 and PAR-6B were upregulated, while the PRKCZ, AKT, and TGF-β1 signaling pathways were activated. Furthermore, rhCOL17 was found to promote cell proliferation and mitigate UV radiation-induced damage, partly by modulating these interacting proteins and their associated signaling pathways. Additional analyses using AlphaFold2 and molecular dynamics simulations revealed that the rhCOL17 peptide bound stably and tightly to the canonical ligand-binding site between the integrin α3 and β1 subunits. These findings highlight the potential versatility and applications of rhCOL17 in the field of antiaging.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


