植物乳杆菌ZUST49中芥子酶介导的硫代葡萄糖苷降解途径的研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
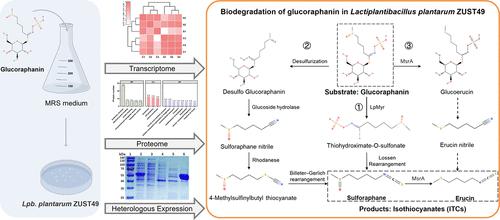
十字花科蔬菜富含硫代葡萄糖苷,可以被黑芥子酶水解成具有显著抗癌特性的异硫氰酸酯(ITCs)。在缺乏细菌黑芥子酶的情况下,硫代葡萄糖苷以无活性形式从体内排出。然而,细菌分解硫代葡萄糖苷的机制尚不清楚。在此,我们研究了益生菌lactoplantibacillus plantarum ZUST49分解硫代葡萄糖苷的机制和酶,该菌将硫代葡萄糖苷中的硫代葡萄糖苷降解为萝卜硫素和芥子素。该菌株的葡萄糖苷降解活性是在葡萄糖苷存在和葡萄糖缺乏的情况下诱导的。降解产物的UPLC-MS分析表明,葡萄糖苷通过三种不同的途径降解,此外,转录组学和蛋白质组学分析鉴定出一个黑芥子酶基因LpMyr,该基因编码460个氨基酸的酶。纯化的LpMyr蛋白在50°C和pH 7.0条件下具有最佳活性,对葡萄糖苷的水解率为7.74 U/mg,对紫荆素的水解率为5.89 U/mg。这些发现为植物乳杆菌的硫代葡萄糖苷转化能力提供了新的见解,并突出了其在发酵工业中高产ITC生产的潜力,以及它在人体肠道中作为益生菌的潜在用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of Myrosinase-Mediated Glucosinolate Degradation Pathways in Lactiplantibacillus plantarum ZUST49
Cruciferous vegetables are rich in glucosinolates that can be hydrolyzed by myrosinase into isothiocyanates (ITCs) with significant anticancer properties. In the absence of bacterial myrosinase, glucosinolates are excreted from the body in their inactive forms. However, the mechanisms underlying the bacterial breakdown of glucosinolates are not well understood. Here, we investigated the mechanism and enzymes involved in glucosinolate breakdown by the probiotic microorganism Lactiplantibacillus plantarum ZUST49, which degrades the glucosinolate glucoraphanin to sulforaphane and erucin. The glucoraphanin-degrading activity of this strain was induced by the presence of glucoraphanin and an absence of glucose. UPLC-MS analysis of the degradation products indicated that glucoraphanin was degraded via three distinct pathways, and further, transcriptomic and proteomic analyses led to the identification of a myrosinase gene, LpMyr, that encodes a 460-amino acid enzyme. The purified LpMyr protein exhibited optimal activity at 50 °C and pH 7.0, with hydrolysis rates of 7.74 U/mg for glucoraphanin and 5.89 U/mg for sinigrin. These findings provide new insights into the glucosinolate conversion capability of L. plantarum and highlight its potential for high-yield ITC production in the fermentation industry, as well as its potential use as a probiotic in the human gut.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


