昆虫Kir通道药理机制及Kir抑制剂对半翅目昆虫毒性的分子研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
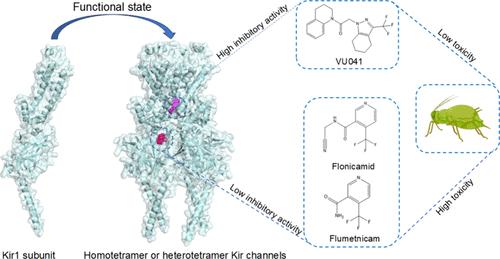
内纠偏钾通道(Kir)在调节多种生理过程中起着关键作用。然而,昆虫Kir通道的结构和药理机制尚不清楚。在这项研究中,我们发现在同一组织中不同Kir亚基的共表达并不影响强整流Kir的整流特性。研究了Kir抑制剂VU041及其代谢物氟硝酰胺(flonicamid)对同四聚体Kir1和Kir2通道的抑制作用。两种昆虫的Kir1和Kir2通道对VU041、氟硝胺和氟美特尼康均表现出相似的药理反应。然而,VU041对所有四个Kir通道的抑制活性明显高于这两种杀虫剂,而氟美替尼康的抑制作用最弱。分子对接分析表明,VU041的结合位点与氟硝胺、氟美尼康不同。flonicamid和flumetnicam的结合位点类似于人类Kir6.2细胞质区域的ATP结合位点,而VU041位于离子通道的孔中,作为孔阻滞剂抑制Kir通道。突变分析证实了这些残基在通道功能和结合亲和力方面的重要作用。最后,评价了3种抑制剂对绿僵菌和桃蚜的毒性。VU041是一种有效的昆虫Kir通道抑制剂,与其他两种抑制剂相比,其毒性较低,而氟甲氧嘧啶对Kir1通道的活性较低,毒性较高,可能与不同化合物的不同生物利用度有关。这些发现表明,靶向Kir通道作为杀虫策略的潜力需要进一步评估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular Insights into Pharmacological Mechanism of Insect Kir Channels and the Toxicity of Kir Inhibitors on Hemipteran Insects
Inwardly rectifying potassium channels (Kir) play a key role in regulating various physiological processes. However, the structural and pharmacological mechanisms of insect Kir channels remain unclear. In this study, we show that coexpression of different Kir subunits in the same tissue did not affect the rectification properties of strongly rectifying Kir. The Kir inhibitor VU041, along with the insecticide flonicamid and its metabolite flumetnicam, were tested for their inhibitory effects on the homotetrameric Kir1 and Kir2 channels. Both Kir1 and Kir2 channels from the two insect species showed similar pharmacological responses to VU041, flonicamid, and flumetnicam. However, VU041 demonstrated significantly higher inhibitory activity than both insecticides across all four Kir channels, while flumetnicam exhibited the weakest inhibition. Molecular docking analyses indicate that the binding site of VU041 is not the same as that of flonicamid, and flumetnicam. flonicamid, and flumetnicam have binding sites similar to the ATP binding sites in cytoplasmic region of human Kir6.2, whereas VU041 is located in the pore of the ion channel, and serves as a pore blocker that inhibits Kir channels. Mutation analysis confirmed the essential roles of these residues in channel function and binding affinity. Finally, the toxicities of the three inhibitors were evaluated in N. lugens and M. persicae. VU041, a potent inhibitor of the insect Kir channel, showed lower toxicity compared to the other two inhibitors, whereas flumethoxan, which is less active on the Kir1 channel, showed higher toxicity, probably related to the different bioavailability of the different compounds. These findings suggest that the potential of targeting Kir channels as insecticidal strategies requires further evaluation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


