肠道苄醚还原酶催化呋喃木脂素高效生物转化为肠木脂素前体的机理
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
肠木脂素是一种重要的抗癌化合物,苯醚还原酶(BER)通过促进木脂素的生物转化在其生物合成中起着关键作用。利用虚拟丙氨酸扫描和定点诱变,我们确定了影响DSM 2243T BER活性的关键残基。突变Y214A、K383A和K395A导致酶活性几乎完全丧失,突出了它们的重要作用。相反,E332Y、G393V和L515A变体的催化效率比野生型BER提高了2倍以上。分子对接和动力学模拟表明,Y214和K383参与底物识别和结合,而K395作为催化碱,形成一个临界η - 3环(残基389-396),调节催化袋的大小和空间阻力。在野生型BER中,该环在底物结合后向内移动5 Å。然而,在E332Y, G393V和L515A突变体中,环分别向外移动4.8,6.1和5.6 Å,可能增强了底物容纳和催化效率。这种η - 3环的运动似乎也影响了氢化物从辅因子向树脂醇的转移,这是催化机制中的关键步骤。这些发现对BER的催化机制提供了有价值的见解,并为优化肠木质素生物制造的酶工程奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
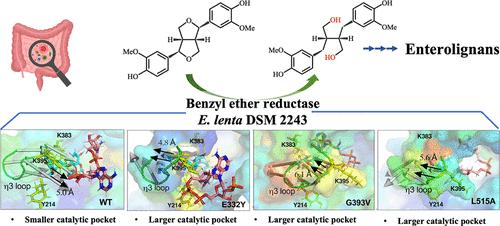
Catalytic Mechanism of Gut Benzyl Ether Reductase for Efficient Bioconversion of Furofuran Lignans into Enterolignan Precursors
Enterolignan is a vital anticancer compound, and benzyl ether reductase (BER) plays a key role in its biosynthesis by facilitating lignan biotransformation. Using virtual alanine scanning and site-directed mutagenesis, we identified critical residues influencing BER activity in DSM 2243T. Mutations Y214A, K383A, and K395A led to a near-complete loss of enzymatic activity, highlighting their essential roles. Conversely, the E332Y, G393V, and L515A variants demonstrated over a 2-fold increase in catalytic efficiency compared to the wild-type BER. Molecular docking and dynamics simulations revealed that Y214 and K383 are involved in substrate recognition and binding, while K395, functioning as a catalytic base, forms a critical η3 loop (residues 389–396) that regulates the catalytic pocket’s size and spatial resistance. In the wild-type BER, this loop moves inward by 5 Å upon substrate binding. However, in the E332Y, G393V, and L515A mutants, the loop shifts outward by 4.8, 6.1, and 5.6 Å, respectively, likely enhancing substrate accommodation and catalytic efficiency. This η3 loop movement also appears to influence hydride transfer from cofactors to pinoresinol, which is a crucial step in the catalytic mechanism. These findings offer valuable insights into BER’s catalytic mechanism and lay a foundation for enzyme engineering to optimize enterolignan biomanufacturing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


