具有良好热稳定性、pH稳定性和广泛醇底物特异性的新型uvarum (Hanseniaspora uvarum, EatH)乙醇乙酰转移酶的表征和机制研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
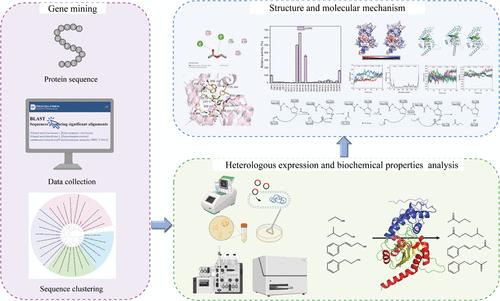
乙酸乙酯是最重要的工业化合物之一,具有广泛的应用,包括香料、香料、制药、化妆品和绿色溶剂。酵母菌中大量生产乙酸乙酯的主要分子是Eat1,但其性质和分子机制尚不清楚。本研究通过基因挖掘的方法,获得了一种来自于兔耳Hanseniaspora uvarum的eat1基因。EatH在pH 7.5和35℃条件下活性最高,优先选择短链酰基底物,但具有从短链伯醇到芳香醇的广泛醇底物谱。对pNPA的Km和kcat/Km值分别为1.16 mM和29.03 L·mmol-1·s-1。EatH的结构由一个盖子结构域和一个核心催化结构域组成,具有Ser124、Asp148和His296的催化三联体。此外,通过分子对接、定点诱变和分子动力学模拟分析了关键残基及其作用机制。突变体N149A、N149K和N149S对pnp -己酸酯的酶活性分别提高了5.0倍、6.6倍和3.6倍,Y204S通过形成更宽的底物结合袋和增强疏水性,使pnp -丁酸酯的酶活性提高了2.6倍。总的来说,这项工作为进一步合理设计earth提供了理论基础,丰富了对Eat家族的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization and Mechanism Study of a Novel Ethanol Acetyltransferase from Hanseniaspora uvarum (EatH) with Good Thermostability, pH Stability, and Broad Alcohol Substrate Specificity
Ethyl acetate, one of the most essential industrial compounds, has a broad range of applications, including flavors, fragrances, pharmaceuticals, cosmetics, and green solvents. Eat1 is accountable for bulk ethyl acetate production in yeasts, yet its properties and molecular mechanism are not well characterized. In this study, an eat1 gene from Hanseniaspora uvarum was obtained through gene mining. EatH showed the highest activity at pH 7.5 and 35 °C and preferred short-chain acyl substrates but had a broad alcohol substrate spectrum from short-chain primary alcohols to aromatic alcohols. Its Km and kcat/Km values toward pNPA were measured to be 1.16 mM and 29.03 L·mmol–1·s–1, respectively. The structure of EatH was composed of a lid domain and a core catalytic domain, with the catalytic triad of Ser124, Asp148, and His296. Additionally, crucial residues and their mechanism were analyzed through molecular docking, site-directed mutagenesis, and molecular dynamics simulation. The mutants N149A, N149K, and N149S showed enhanced enzyme activity toward pNP-hexanoate to 5.0-, 6.6-, and 3.6-fold, and Y204S enhanced enzyme activity for pNP-butyrate by 2.6 times via creating a wider substrate binding pocket and enhancing hydrophobicity. Collectively, this work provided a theoretical basis for the further rational design of EatH and enriched the understanding of the Eat family.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


