IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本研究调查了未改性/改性(球磨法,BMP;高压匀浆法,HPHP;冷等离子体法,CPP)香附蛋白(CEP)与原花青素(PA 和 PB2)之间的非共价相互作用,以评估其在乳液中的结构、功能和潜力。加入 PA 和 PB2 后,CEP 样品的浊度和ζ电位明显增加,原子力显微镜观察到的聚集现象也证实了这一点,从而验证了蛋白质-原花青素复合物的形成。荧光淬灭和等温滴定量热法显示,原花青素导致CEP样品静态淬灭,CEP-原花青素的结合亲和力顺序为CPP > HPHP> BMP > CEP。CEP-原花青素在氢键和静电作用的驱动下进行非共价相互作用,不会改变CEP样品的光谱带和二级结构,但会增强热稳定性、抗氧化活性和乳化性能。然后,CPP-PA 稳定的乳液液滴尺寸随着水相 pH 值的增加而减小,这与ζ电位值相反。总之,这些研究结果表明,改性 CEP-原花青素复合物是应对这些挑战和稳定乳液的一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
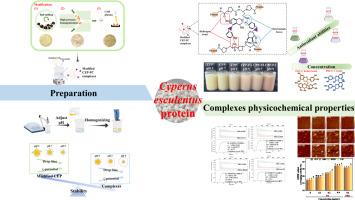
Three physical modifications enhanced the binding interactions of Cyperus esculentus protein with proanthocyanidins and physicochemical properties of complexes: The contribution of non-covalent interactions
This study investigated non-covalent interactions between unmodified/modified (ball-milling, BMP; high pressure homogenization, HPHP; cold plasma, CPP) Cyperus esculentus protein (CEP) and proanthocyanidins (PA and PB2) to evaluate structure, functionalities and potential in emulsions. The PA and PB2 addition significantly increased the turbidity and ζ-potential of CEP samples, as confirmed by aggregations observed via atomic force microscopy, validating the formation of protein-proanthocyanidin complexes. Fluorescence quenching and isothermal titration calorimetry revealed that procyanidins caused CEP sample static quenching, with CEP-proanthocyanidins binding affinity order as CPP > HPHP>BMP > CEP. The CEP-proanthocyanidins involve non-covalent interactions, driven by hydrogen bonding and electrostatic interactions, without altering CEP sample spectral bands and secondary structures, but enhancing thermal stabilities, antioxidant activities, and emulsifying properties. Then, the CPP-PA stabilized emulsion droplet size decreased with aqueous phase pH increasing, contrary to ζ-potential values. Conclusively, these findings illustrated that the modified CEP-proanthocyanidin complexes as a promising strategy for addressing these challenges and stabilizing emulsion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


