猪笼草蛋白酶的裂解特异性及其对牛奶蛋白致敏性的降低
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本研究以猪笼草(Nepenthes × miranda)的猪笼液蛋白酶作为一种新的蛋白酶资源进行研究,因为它具有裂解特异性和降低牛奶蛋白的致敏性的能力。我们发现这些蛋白酶在含有K、L、V、S、I和R残基的P1位点特别有效,并且与P1位点的氨基酸残基表现出相似的偏好。由此可见,P1负责猪笼液蛋白酶的特异性,而P1 '在水解过程中倾向于表现出其广泛性。与表位的破坏一致,体内试验也表明,乳清蛋白浓缩物和酪蛋白都能降低致敏性,尽管乳清蛋白浓缩物对酪蛋白的影响减弱。因此,这些蛋白酶具有巨大的潜力,值得进一步开发用于解决牛奶蛋白过敏的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
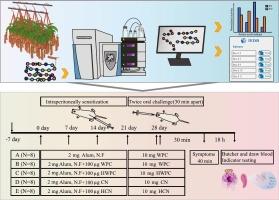
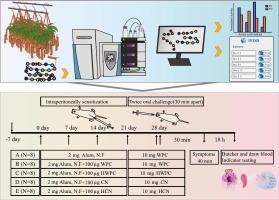
Cleavage specificity of the pitcher fluid proteases from Nepenthes × miranda and their reduction on allergenicity of cow's milk proteins
In this study, the pitcher fluid proteases from Nepenthes × miranda were researched as a novel protease resource due to their cleavage specificity and ability to reduce the allergenicity of cow's milk proteins. We found that these proteases are particularly efficient at the P1 position with K, L, V, S, I, and R residues and exhibit similar preferences to amino acid residues at the P1′ position. It is concluded that P1 is responsible for specificity of pitcher fluid proteases, while P1′ tends to show their broadness when hydrolyzation happens. And consistent with the destroying of epitopes, in vivo assays also demonstrated a reduction in allergenicity from both whey protein concentrates and caseins, although the effect on caseins paled to whey protein concentrates. Therefore, these proteases hold significant potential and warrant further development for applications addressing cow's milk protein allergies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


