新型2-(1h -吲哚-2-基)-1,3,4-恶二唑衍生物对灰霉病菌的抑菌活性及初步作用模式研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
新先导物和新靶点的发现是新杀菌剂开发解决杀菌剂耐药性问题的重要途径。吲哚骨架因其独特的生物活性在农用化学品中得到了广泛的应用。YZK-C22是一种有效的丙酮酸激酶抑制剂,具有很高的抗真菌活性。在YZK-C22的基础上开发了几种新型杀菌剂先导剂。受此启发,[1,2,4]三嗪基[4,5-a]吲哚-1(2H)- 1衍生物采用跳骨架策略设计;然而,这些化合物表现出中等的杀真菌活性。出乎意料的是,在控制反应条件下生成的2-(1h -吲哚-2-基)-1,3,4-恶二唑衍生物的杀真菌活性显著提高。化合物6c、6d、6f和6j对灰霉病菌的EC50值为0.120 ~ 0.310 μg/mL,比市售杀菌剂嘧霉胺(EC50 = 0.990 μg/mL)的抑菌活性更强。在540和720 g活性成分(ai)/hm2的田间试验中,化合物6c对灰绿杆菌的防治效果分别为81.46%和86.58%,高于嘧菌胺540 gai /hm2的防治效果(70.46%)。与YZK-C22相比,化合物6c和6d对葡萄球菌丙酮酸激酶的亲和常数较低。较高的场效,但较低的亲和力丙酮酸激酶表明,这些化合物可能作为前药或具有不同的作用模式。因此,2-(1h -吲哚-2-基)-1,3,4-恶二唑衍生物值得进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
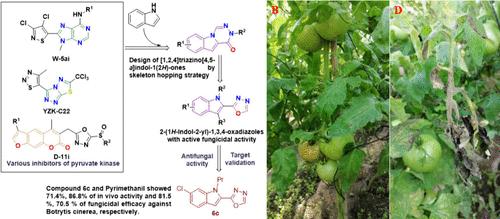
Investigation of the Antifungal Activity and Preliminary Mode of Action of Novel 2-(1H-Indol-2-yl)-1,3,4-oxadiazole Derivatives against Botrytis cinerea
The discovery of novel leads and new targets is an important approach to address the issue of fungicide resistance by new fungicide development. The indole skeleton has been widely utilized in agrochemicals due to its unique biological activity. YZK-C22 is a potent pyruvate kinase inhibitor with high antifungal activity. Several novel fungicide leads were developed based on YZK-C22. Inspired by these, the [1,2,4]triazino[4,5-a]indol-1(2H)-one derivatives were designed using a skeleton hopping strategy; however, these compounds exhibited moderate fungicidal activity. Unexpectedly, 2-(1H-indol-2-yl)-1,3,4-oxadiazole derivatives formed under controlled reaction conditions showed significantly higher fungicidal activity. Compounds 6c, 6d, 6f, and 6j exhibited excellent antifungal activity in vitro, with EC50 values ranging from 0.120 to 0.310 μg/mL against Botrytis cinerea, more potent than commercial fungicide pyrimethanil (EC50 = 0.990 μg/mL). In the field trials at 540 and 720 g of active ingredient (ai)/hm2, compound 6c exhibited 81.46 and 86.58% efficacy against B. cinerea, higher than that of pyrimethanil at a rate of 540 g of ai/hm2 (70.46%). The affinity constants of compounds 6c and 6d to pyruvate kinase from B. cinerea were lower than that of YZK-C22. Higher field efficacy but lower affinity to pyruvate kinase implies that these compounds may work as prodrugs or have a different mode of action. Thus, 2-(1H-indol-2-yl)-1,3,4-oxadiazole derivatives are worth being further investigated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


