IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
近年来,熵驱动电路(EDC)动态 DNA 网络因其简单、高效和设计灵活而在核酸检测领域备受关注。然而,传统的 EDC 反应由于驱动力单一且微弱,在实现最佳信号放大方面面临着限制。为了克服这一限制,我们创新性地设计了一种金纳米粒子(AuNP)分散增强 EDC(Au-EDC)方法,开创了一种新型比色信号放大和输出系统。该系统与连接酶链反应(LCR)和谐地结合在一起,用于精确的单核苷酸多态性(SNP)基因分型。具体来说,LCR 只在突变目标(MT)的阳性链上选择性地执行,从而促进了精确的点到链信息转导。随后,LCR 产物触发 Au-EDC 循环反应,使 DNA-AuNPs 网络逐渐解体并释放出明显的比色信号。这一战略性设计巧妙地利用了 AuNPs 从聚集状态过渡到分散状态时发生的熵增加,为 EDC 循环提供了补充动力。LCR-Au-EDC 集成系统在检测浓度低至 320 fM 的 MT 和区分突变频率低至 0.1% 的集合样本方面表现出色。此外,该系统还能对来自大豆叶片的真实基因组进行准确的 SNP 基因分型。因此,本研究不仅开发了一种基于 EDC 反应的比色信号放大和输出传感系统,还提供了一种经济高效的 SNP 基因分型工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
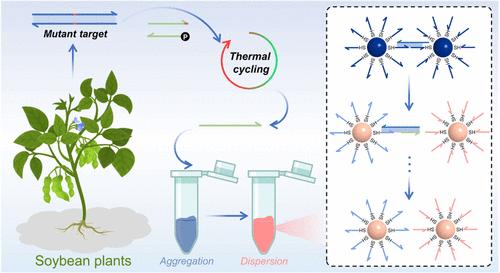
Entropy-Driven Circuit Integrated with Ligases to Regulate DNA-AuNP Network Disintegration for Colorimetric Detection of Single Nucleotide Polymorphisms
In recent years, entropy-driven circuit (EDC) dynamic DNA networks have garnered significant attention in nucleic acid detection owing to their simplicity, efficiency, and flexible design. Nevertheless, conventional EDC reactions face a constraint in achieving optimal signal amplification due to a solitary and feeble driving force. To overcome this limitation, we innovatively devised a gold nanoparticle (AuNP) dispersion-enhanced EDC (Au-EDC) approach, pioneering a novel colorimetric signal amplification and output system. The system was harmoniously integrated with the ligase chain reaction (LCR) for precise single nucleotide polymorphism (SNP) genotyping. Specifically, LCR was selectively executed solely on the positive strand of the mutant target (MT), facilitating precise point-to-strand information transduction. Subsequently, the LCR product triggered the Au-EDC cycling reaction, causing the DNA-AuNPs network to progressively disintegrate and release a pronounced colorimetric signal. This strategic design ingeniously harnessed the entropy increase that occurs as AuNPs undergo a transition from aggregated to dispersed states, offering a supplemental impetus for the EDC cycle. The integrated LCR-Au-EDC system excelled in detecting MT at concentrations as low as 320 fM and differentiating pooled samples with mutation frequencies as low as 0.1%. Moreover, the system accurately performed SNP genotyping on the real genomes derived from soybean leaves. Consequently, this study not only develops a colorimetric signal amplification and output sensing system based on EDC reactions but also provides a cost-effective and efficient SNP genotyping tool.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


