燕麦β-葡聚糖增强了肌纤维蛋白乳的稳定性和流变特性:对结构和界面相互作用的见解
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
研究了燕麦β-葡聚糖(OG)对海鲈肌纤维蛋白(MP)的结构、界面行为和乳液流变特性的影响。傅立叶变换红外光谱显示,加入 OG 后,MP 中的α-螺旋含量从 27% 降至 22%,而β-片状含量则有所增加,这表明 OG 有助于 MP 的展开。当 OG 含量为 1.2% 时,界面张力降低了约 20%,扩散速率增加了约 1.5 倍。这些变化增强了乳液的物理稳定性。流变分析表明,3% 的 OG 提高了乳液的储能模量(G')。这些变化增强了 MP 在油水界面的吸附和重排,从而增强了界面薄膜的粘弹性,进一步提高了乳液的稳定性。这些发现加深了人们对蛋白质与多糖相互作用的理解,并为提高蛋白质乳液稳定性提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。

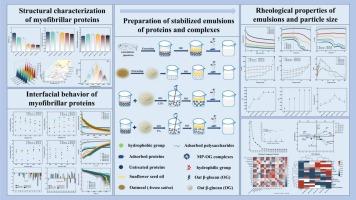
Enhanced stability and rheological properties of myofibrillar proteins emulsions conferred by oat β-glucan: Insights into structural and interfacial interactions
The effect of oat β-glucan (OG) on the structure, interfacial behavior and emulsion rheological properties of sea bass myofibrillar protein (MP) was investigated. Fourier transform infrared spectroscopy demonstrated that adding OG decreased the α-helix content in MP from 27% to 22% and increased the β-sheet content, suggesting that OG facilitated MP unfolding. When the OG content was 1.2%, the interfacial tension decreased by approximately 20%, and the diffusion rate increased by approximately 1.5-fold. These changes augmented the physical stability of emulsions. Rheological analysis showed that 3% OG promoted the energy storage modulus (G') of the emulsion. These changes enhanced MP adsorption and rearrangement at the oil-water interface, which enhanced viscoelasticity in the interfacial film and further increased the emulsion stability. These findings augment the understanding of protein-polysaccharide interactions and provide guidance for improving protein emulsion stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


