超声和脉冲电场预处理小粉虫蛋白水解物稳定5 %鱼油水乳状液的物理和氧化稳定性
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
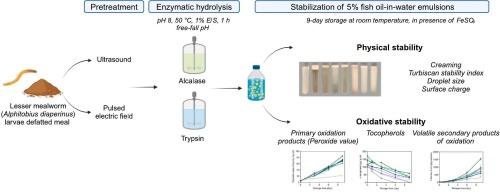
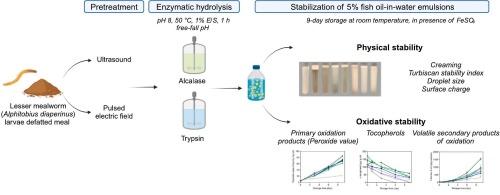
采用超声(US)或脉冲电场(PEF)预处理小粉虫(Alphitobius diaperinus幼虫)的饲料,并用Alcalase或胰蛋白酶进行水解。所得到的水解液被评价其维持5 %鱼油水乳状液的物理和氧化稳定性的能力。通过测定酶解度(DH)、蛋白产量和分子量分布来评价预处理对酶解的影响。水解产物DH值为19-28 %。通过乳化指数、Turbiscan稳定性指数、ζ-电位和液滴尺寸来评价物理稳定性。经us预处理的胰蛋白酶水解物稳定的乳状液液滴尺寸最小(0.626 μm)。在贮存过程中测定一次氧化产物和挥发性二次氧化产物。然而,没有一种水解物稳定的乳剂表现出比酪蛋白酸钠(参比蛋白)更高的氧化稳定性。这些结果表明,尽管经us预处理的胰蛋白酶水解物具有作为乳化剂的潜力,但还需要额外的抗氧化剂来有效控制脂质氧化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Physical and oxidative stability of 5 % fish oil-in-water emulsions stabilized with lesser mealworm (Alphitobius diaperinus larva) protein hydrolysates pretreated with ultrasound and pulsed electric fields
Lesser mealworm (Alphitobius diaperinus larva) meal was pretreated with ultrasound (US) or pulsed electric fields (PEF) and hydrolyzed using Alcalase or Trypsin enzymes. The resulting hydrolysates were evaluated for their ability to maintain physical and oxidative stability of 5 % fish oil-in-water emulsions. The effects of the pretreatment on enzymatic hydrolysis were assessed by measuring the degree of hydrolysis (DH), protein yield, and molecular weight distribution. Hydrolysates with 19–28 % DH were produced. Physical stability was evaluated in terms of creaming index, Turbiscan stability index, ζ-potential, and droplet size. Emulsions stabilized with US-pretreated Trypsin hydrolysates presented the smallest droplet sizes (0.626 μm). Primary and volatile secondary oxidation products were measured during storage. However, none of the hydrolysate-stabilized emulsions exhibited greater oxidative stability than sodium caseinate, the reference protein. These results suggest that although US-pretreated Trypsin hydrolysates exhibit potential as emulsifiers, additional antioxidants are needed to effectively control lipid oxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


