菊糖果糖转移酶制备二果糖酸酐I的理化性质
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
尽管假设了二果糖酸酐I (DFAI)的新功能,但其物理化学特征仍然不够充分。本文研究了利用菊粉果糖转移酶酶法合成菊粉dfa - 1的性质。通过13C核磁共振和质谱分析验证了其结构。进一步的分析结果表明,DFA-I的旋光度aD20aD20为+28.12 ± 0.08°,熔点为163.4 °C,具有优异的热稳定性(≤250 °C)。在10 mg·mL−1时,化合物的电导率为0.5 ± 0.10 μS·cm−1。dfa - 1在极端酸性条件下(pH 2.0,100 °C, 30 min)保持97.07 %的稳定性,在长时间暴露(pH 3.0,37 °C, 3 个月)保持95.07 %的稳定性。该化合物具有可忽略的美拉德反应活性(A420 ≤ 0.050,pH值为 3.0-8.0),显著的亲水性(溶解度:300 g·(100 g H₂O)−1,20 °C),在75-94 %相对湿度(25 °C)下具有明显的吸湿性。这些发现为dfa - 1的功能探索和工业应用奠定了严谨的理化基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
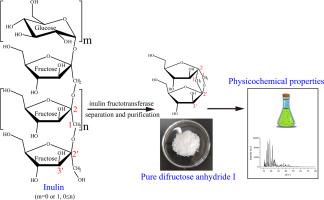
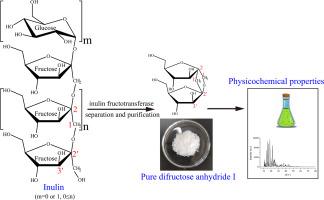
Physical and chemical properties of difructose anhydride I produced from inulin by inulin fructotransferase
Despite the hypothesized novel functionalities of difructose anhydride I (DFA-I), its physicochemical profile remains insufficiently characterized. This study elucidated the properties of enzymatically synthesized DFA-I derived from inulin using inulin fructotransferase. Structural elucidation via 13C NMR and mass spectrometry validated its identity. Further analytical results demonstrated that DFA-I exhibited an optical rotation of +28.12 ± 0.08°, a melting point of 163.4 °C, and exceptional thermostability (≤250 °C). At 10 mg·mL−1, the compound displayed a conductivity of 0.5 ± 0.10 μS·cm−1. DFA-I retained 97.07 % stability under extreme acidic conditions (pH 2.0, 100 °C, 30 min) and 95.07 % stability during prolonged exposure (pH 3.0, 37 °C, 3 months). The compound exhibited negligible Maillard reactivity (A420 ≤ 0.050 across pH 3.0–8.0), remarkable hydrophilicity (solubility: 300 g·(100 g H₂O)−1 at 20 °C), and pronounced hygroscopicity under 75–94 % relative humidity (25 °C). These findings establish a rigorous physicochemical foundation for DFA-I's functional exploration and industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


