酸可切割三磷酸鸟苷-光亲和探针用于三磷酸鸟苷结合蛋白及其活性位点的全局分析
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
鸟苷三磷酸(GTP)结合蛋白在细胞信号传导中起着分子开关的作用,在多种生物通路中发挥着重要作用。它们的失调与许多疾病的起因和进展有关。对gtp结合蛋白的系统分析将有助于相关信号通路和药物的研究。先前报道的酰基磷酸gtp亲和探针,与gtp结合口袋附近的赖氨酸残基反应并标记,已被证明在识别标记位点方面是有效的,但由于其高反应性,稳定性较差。我们在这里报道了新的gtp光亲和探针,该探针采用紫外线触发的光反应基团用于蛋白质的共价标记,大大提高了探针的稳定性。末端炔基的包含允许标记的蛋白质用荧光团进行荧光分析或用生物素组进行lc -质谱(MS)/MS分析。我们进一步设计了一种具有酸可切割P-N键的GTP-N探针。在标记和富集后的酸性条件下孵育,P-N键使GTP从标记位点释放,从而减少了蛋白质修饰的质量位移,促进了基于ms的修饰位点鉴定。该方法在鉴定新的gtp结合蛋白和系统分析gtp结合位点方面具有良好的潜力。这些新型的gtp光亲和探针可进一步应用于gtp酶抑制剂的生化机制研究和评价。本文章由计算机程序翻译,如有差异,请以英文原文为准。
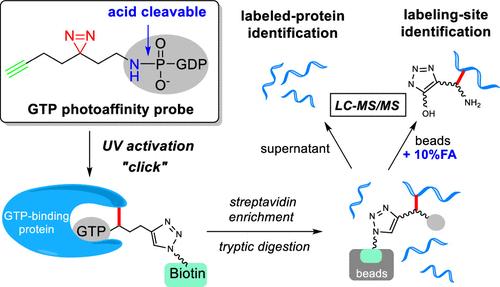
Acid-Cleavable Guanosine Triphosphate-Photoaffinity Probe for Global Profiling of Guanosine Triphosphate-Binding Proteins and Their Active Sites
Guanosine triphosphate (GTP)-binding proteins function as molecular switches in cell signaling, playing critical roles in various biological pathways. Their dysregulation is associated with the causes and progression of many diseases. Systematic analysis of GTP-binding proteins would facilitate studies of related signaling pathways and drugs. Previously reported acyl-phosphate GTP-affinity probes, which react with and label lysine residues near GTP-binding pockets, have proven efficient in identifying labeling sites but suffer from poor stability due to their high reactivity. We report here new GTP-photoaffinity probes that employ a UV-triggered photoreactive group for covalent labeling of proteins, greatly improving probe stability. The inclusion of a terminal alkyne group allows labeled proteins to be tagged either with a fluorophore for fluorescence analysis or with a biotin group to enrich for LC–mass spectrometry (MS)/MS analysis. We further designed a GTP-N probe featuring an acid-cleavable P–N bond. The P–N bond enabled the release of GTP from labeling sites upon incubation under acidic conditions after labeling and enrichment, which reduced protein-modification mass shift and facilitated MS-based modification-site identification. This new method demonstrates good potential for identifying new GTP-binding proteins and systematically analyzing GTP-binding sites. These novel GTP-photoaffinity probes could be further applied in studying related biochemical mechanisms and in evaluating GTPase inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


