多环连接促进的指数重组酶扩增平台的构建,用于敏感和同时监测癌症生物标志物Fpg和FEN1
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
甲脒嘧啶DNA糖基化酶(Fpg)和皮瓣内切酶1 (FEN1)对维持基因组的稳定性和完整性至关重要,而Fpg和FEN1的异常活动可能导致多种疾病和癌症。开发同时监测Fpg和FEN1的简单方法是非常必要的。在此,我们构建了一个多环连接促进的指数重组酶聚合酶扩增(RPA)平台,用于敏感和同时监测细胞和临床组织中的Fpg和FEN1。我们设计了两个可编程底物探针,具有8-oxo-7,8-二氢鸟嘌呤(8-oxoG)损伤位点和5 '襟翼,可以被Fpg和FEN1识别/切割以产生缺口位点。并置的切割位点通过DNA连接酶连接形成完整的双链DNA (dsDNA)模板,可以通过RPA扩增产生丰富的dsDNA产物,分别标记为Cy5和Cy3荧光团和生物素。所得的dsDNA可以被磁珠捕获,随后在热处理后分解成分散的Cy3和Cy5分子,产生显著的荧光信号。该方法对Fpg和FEN1的检出限分别为1.12 × 10-10 U μL-1和1.66 × 10-9 U μL-1,可用于酶动力学参数分析、抑制剂筛选、单细胞和临床组织样品中Fpg和FEN1的同时监测。此外,该策略可以通过改变dsDNA底物探针的识别位点来监测其他DNA修复蛋白,为临床诊断、生物医学研究和药物发现提供了一个有前景的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
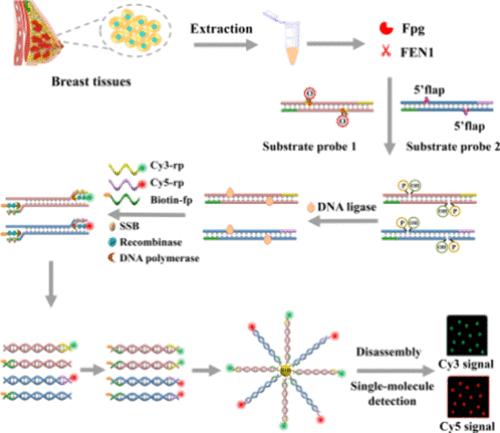
Construction of a Multiple Cyclic Ligation-Promoted Exponential Recombinase Polymerase Amplification Platform for Sensitive and Simultaneous Monitoring of Cancer Biomarkers Fpg and FEN1
Formamidopyrimidine DNA glycosylase (Fpg) and flap endonuclease 1 (FEN1) are essential to sustaining genomic stability and integrity, while the abnormal activities of Fpg and FEN1 may lead to various diseases and cancers. The development of simple methods for simultaneously monitoring Fpg and FEN1 is highly desirable. Herein, we construct a multiple cyclic ligation-promoted exponential recombinase polymerase amplification (RPA) platform for sensitive and simultaneous monitoring of Fpg and FEN1 in cells and clinical tissues. We designed two programmable substrate probes with 8-oxo-7,8-dihydroguanine (8-oxoG) damage sites and 5′ flaps that can be identified/cleaved by Fpg and FEN1 to produce nicking sites. The juxtaposition of the cleavage sites is ligated by DNA ligase to form intact double-stranded DNA (dsDNA) templates that can be amplified via RPA to produce abundant dsDNA products labeled with Cy5 and Cy3 fluorophores and biotin, respectively. The resultant dsDNA can be captured by magnetic beads and subsequently disassembled into dispersed Cy3 and Cy5 molecules upon heat treatment, generating significant fluorescence signals. This assay exhibits a limit of detection of 1.12 × 10–10 U μL–1 for Fpg and 1.66 × 10–9 U μL–1 for FEN1, and it can be used for the analysis of enzymatic kinetic parameters, screening of inhibitors, and simultaneous monitoring of Fpg and FEN1 in a single cell and in clinic tissue samples. Moreover, the proposed strategy can be applied to monitor other DNA repair proteins by merely changing the recognition sites of dsDNA substrate probes, providing a promising platform for clinical diagnosis, biomedical research, and drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


