IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
氨基酸脱氢酶对 l-苯基甘氨酸的酶促不对称合成具有工业应用潜力;然而,由于它们对高浓度底物的催化效率较低,这一点受到了阻碍。我们通过定向元基因组学方法发现并鉴定了一种新型亮氨酸脱氢酶(MsLeuDH),它对苯甲酰甲酸具有很高的催化效率。此外,我们还通过合理设计远端环 13 获得了一个三点突变体 MsLeuDH-EER(D332E/G333E/L334R),其稳定性和催化效率均有所提高。MsLeuDH-EER 和甲酸脱氢酶的共表达系统在 4 小时内完全转化了 300 mM 的底物,对映体过量率达 99.9%。分子动力学模拟显示,环 13 上的突变增强了蛋白质的整体结构刚性,从而提高了其稳定性,而且还通过远端调控使铰链区环刚性化,从而稳定了 "封闭 "构象。我们的研究结果表明,远端环 13 可以作为提高亮氨酸脱氢酶催化性能的新热点区域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
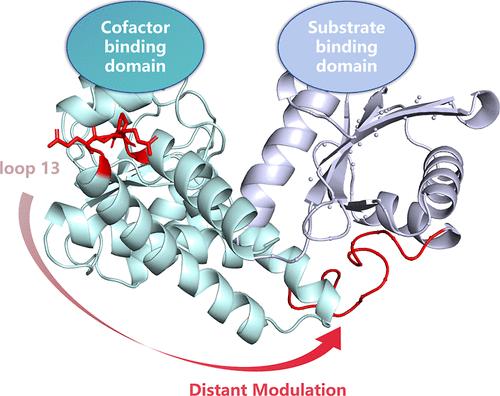
Semirational Engineering of a Distal Loop Region to Enhance the Catalytic Activity and Stability of Leucine Dehydrogenase
Enzymatic asymmetric synthesis of l-phenylglycine by amino acid dehydrogenases has potential for industrial applications; however, this is hindered by their low catalytic efficiency toward high-concentration substrates. We identified and characterized a novel leucine dehydrogenase (MsLeuDH) with a high catalytic efficiency for benzoylformic acid via directed metagenomic approaches. Further, we obtained a triple-point mutant MsLeuDH-EER (D332E/G333E/L334R) with improved stability and catalytic efficiency through the rational design of distal loop 13. A coexpression system of MsLeuDH-EER and formate dehydrogenase completely converted a 300 mM substrate within 4 h with >99.9% enantiomeric excess. Molecular dynamics simulations revealed that mutations on loop 13 enhanced the overall structural rigidity of the protein to improve its stability but also stabilized the “closed” conformation through rigidifying the hinge region loop by distant modulation. Our results show that distal loop 13 can serve as a new hotspot region for enhancing the catalytic performance of leucine dehydrogenases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


