紫丁香酸甲酯单葡萄糖苷是钩端精子生物合成的重要中间体
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
Leptosperin(甲基丁香-4- o -β-d-gentiobioside)是ma′s nuka蜂蜜的独特标记物,它是从ma′s nuka植物(Leptospermum scoparium)中提取的。尽管瘦精子素具有重要意义,但其生物合成途径尚未见报道。本文研究了麻麻属植物丁香酸甲酯(MSYR)苷元形成细精素的分子机制。在马乌卡花蜜中鉴定出紫丁香甲基-4- o -β-d-葡萄糖(MSYR-glucose),而在马乌卡蜂蜜中未鉴定出。MSYR分布在麻麻植物的花、叶、枝和根中,而MSYR-葡萄糖和lepptosperin仅在花中存在。通过将切花枝浸泡在氘标记的水介质中,分析了氘化瘦精素(leptosperin-d6)和msyr -葡萄糖(MSYR-d6-glucose)的形成。当加入MSYR-d6时,同时检测MSYR-d6-葡萄糖和瘦精素-d6。添加合成的MSYR-d6-葡萄糖也会生成瘦精子素-d6,这表明瘦精子素中的龙胆苷部分是通过MSYR与d-葡萄糖结合,然后再添加另一个d-葡萄糖形成的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
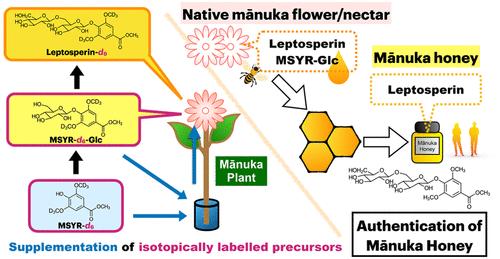
Methyl Syringate Monoglucoside Is a Crucial Intermediate in Leptosperin Biosynthesis in Leptospermum scoparium (ma̅nuka)
Leptosperin (methyl syringate-4-O-β-d-gentiobioside) serves as a unique marker for ma̅nuka honey, derived from the ma̅nuka plant (Leptospermum scoparium). Despite its importance, the biosynthesis pathway of leptosperin remains unreported. This study investigates the molecular mechanism of leptosperin formation from its aglycone, methyl syringate (MSYR), in ma̅nuka plants. Methyl syringate-4-O-β-d-glucopyranoside (MSYR-glucose) was identified in ma̅nuka flower nectar but not in ma̅nuka honey. MSYR was distributed in the flowers, leaves, branches, and roots of ma̅nuka plants, while MSYR-glucose and leptosperin were only observed in the flowers. By immersing a cut flowering branch in a deuterium-labeled aqueous medium, the formation of deuterated leptosperin (leptosperin-d6) and MSYR-glucose (MSYR-d6-glucose) was analyzed. When MSYR-d6 was added, both MSYR-d6-glucose and leptosperin-d6 were detected. Supplementation with synthetic MSYR-d6-glucose also generated leptosperin-d6, indicating that gentiobioside moiety in leptosperin forms through the conjugation of MSYR with d-glucose, followed by the addition of another d-glucose.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


