表没食子儿茶素-3-没食子酸盐负载金属-有机框架防止凡纳滨对虾鱼糜凝胶氧化降解的机制
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
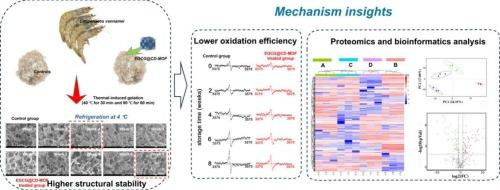
本研究旨在阐明虾鱼腥凝胶在冷藏过程中的变质机制,以及表没食子儿茶素-3-没食子酸酯负载环糊精基金属有机骨架(EGCG@CD-MOF)作为模型抗氧化剂的调控机制。无标签蛋白质组学提供了对降解蛋白质的差异蛋白质组学特征的定量分析。结构蛋白,如肌凝蛋白、副肌凝蛋白、肌动蛋白、层粘连蛋白和α-肌动蛋白,以及钙调节蛋白,如钙调磷酸酶和肌浆钙结合蛋白,被发现在冷藏过程中极易氧化降解。相反,EGCG@CD-MOF显著减轻了蛋白质的降解。电子自旋共振(ESR)数据表明,EGCG@CD-MOF在8周的冷藏期间有效地抑制了自由基的积累。扫描电镜(SEM)进一步证实了其防止网状结构劣化的能力。此外,流变学、红外和分子动力学分析支持EGCG@CD-MOF与蛋白质之间的持续相互作用,确定了ASP-131、ARG-92、SER-97、ASP-98、Lys-12、Gly-168、Glu-170、Arg-8和Gly-6残基上的关键相互作用位点。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Mechanisms of epigallocatechin-3-gallate-loaded metal−organic framework in preventing oxidative degradation of shrimp (Litopenaeus vannamei) surimi gel
This work aimed to elucidate the deterioration mechanisms of shrimp surimi gels during refrigerated storage, and the regulatory mechanisms of epigallocatechin-3-gallate loaded cyclodextrin-based metal−organic framework (EGCG@CD-MOF) as a model antioxidant. Labele-free proteomics provided a quantitative analysis of the differential proteomic signatures of degraded proteins. Structural proteins, like myosin, paramyosin, titin, laminin, and α-actinin, along with calcium regulatory proteins, like calcineurin and sarcoplasmic calcium-binding protein were found to be highly susceptible to oxidative degradation during refrigeration. In contrast, EGCG@CD-MOF significantly mitigated protein degradation. Electron spin resonance (ESR) data demonstrated that EGCG@CD-MOF efficiently inhibited free radical accumulation over the 8-week refrigeration period. Scanning electron microscopy (SEM) further confirmed its ability to prevent network structural deterioration. Additionally, rheological, infrared, and molecular dynamics analyses supported the sustained interaction between EGCG@CD-MOF and proteins, with key interaction sites identified at residues ASP-131, ARG-92, SER-97, ASP-98, Lys-12, Gly-168, Glu-170, Arg-8, and Gly-6.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


