手性农药艾替普洛及其手性代谢物艾替普洛酰胺在5种蔬菜中的对映选择行为及风险评价
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
乙虫腈是第二代苯吡唑类杀虫剂,由于其对蜜蜂的毒性较低,在农业生产中被用作氟虫腈的替代品。乙替普罗胺是乙替普罗的手性代谢物,但有关其在蔬菜中的形成和降解的信息有限。本文通过圆二色性测定了乙替普罗胺对映体的绝对构型,并通过田间试验研究了手性乙替普罗及其代谢物在5种蔬菜中的行为。在这些试验蔬菜中,乙普罗胺的浓度高于乙普罗胺砜酰胺,其中芹菜的浓度最高。s -乙丙酰胺在小白菜和大白菜中被优先降解,且r -乙丙酰胺浓度高于s -乙丙酰胺。考虑到人类可能暴露于特定对映体的高残留水平,对对映体特异性行为的评估至关重要。在评估与手性农药及其代谢物相关的风险时,应考虑对映体选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

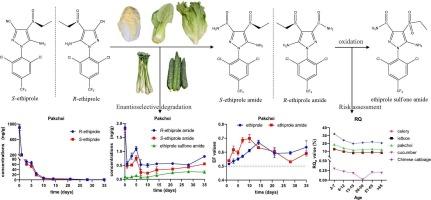
Enantioselection behaviors and risk assessments of chiral pesticide ethiprole and its chiral metabolite ethiprole amide in five kinds of vegetables
Ethiprole is a second-generation phenylpyrazole insecticide used in agricultural production as an alternative to fipronil due to its lower toxicity to bees. Ethiprole amide is chiral metabolite of ethiprole, but information regarding its formation and degradation in vegetables is limited. Here, the absolute configuration of ethiprole amide enantiomer was determined through circular dichroism, and the behaviors of chiral ethiprole and its metabolites in five kinds of vegetables were studied through field experiments. In these test vegetables, ethiprole amide concentrations were higher than ethiprole sulfone amide, with the maximum concentration found in celery. S-ethiprole was preferentially degraded in pakchoi and Chinese cabbage, and R-ethiprole amide concentration exceeded S-ethiprole amide. This assessment of enantiomer-specific behavior is crucial, considering that humans may be exposed to high residual levels of a specific enantiomer. Enantioselectivity should be taken into account when evaluating the risks associated with chiral pesticides and their metabolites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


