纳米孔中β-发夹肽的复杂和非顺序电流特征
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
纳米孔传感广泛应用于单分子检测,最初应用于核酸检测,现已扩展到蛋白质检测。我们的研究重点是肽在纳米孔中的复杂构象变化,这可能对肽指纹图谱和蛋白质鉴定具有重要意义。具体来说,我们研究了β-发夹肽(SV28)在α-溶血素(αHL)纳米孔中的相互作用。我们的实验表明,SV28通过介电泳捕获,并在纳米孔内停留时间长,导致多种电流阻断水平。与DNA发夹不同,该肽在四个不同的阻断水平之间表现出非顺序转变。这种复杂的行为表明,纳米孔中的肽动力学不能简单地沿单一反应坐标建模。我们的发现提供了对肽-纳米孔相互作用的见解,这对开发基于纳米孔的肽鉴定技术可能有用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
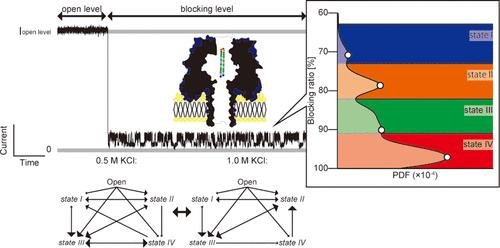
Complex and Non-sequential Current Signatures of a β-Hairpin Peptide Confined in a Nanopore
Nanopore sensing is widely used for single-molecule detection, originally applied to nucleic acids and now extended to protein sensing. Our study focuses on the complex conformational changes of peptides in nanopores, which may have implications for peptide fingerprinting and protein identification. Specifically, we investigated the interaction of a β-hairpin peptide (SV28) within an α-hemolysin (αHL) nanopore. Our experiments revealed that SV28 is captured via dielectrophoresis and exhibits long dwell times within the nanopore, leading to multiple current blockade levels. Unlike DNA hairpins, the peptide showed non-sequential transitions among four distinct blockade levels. This complex behavior indicates that the peptide dynamics in nanopores cannot be simply modeled along a single reaction coordinate. Our findings provide insights into peptide-nanopore interactions, which are potentially useful for developing nanopore-based peptide identification technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


