π共轭桥增强α-甲基色胺特异性检测的精确吸电子强度调节
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
α-甲基色胺(AMT)的特异性荧光检测具有很大的挑战性,因为在多种胺中存在相似的胺基和苯环。在这里,我们展示了在含醛的希夫碱荧光探针中π共轭桥的吸电子强度的精确调制,用于超痕量AMT识别。发现- C6H4、- C6H2N2和- C6H2Br2等不同的吸电子基团作为2-二氰甲基-3-氰基-4,5,5-三甲基-2,5-二氢呋喃(TCF)探针的π共轭桥,可以对具有不同亲核性的有机胺进行分类和鉴定。值得注意的是,以−C6H2Br2为π共轭桥的探针(表示为BrFS-TCF)具有最高的识别位点亲电性,对AMT具有更高的nm级检测限(LOD)和即时响应时间(<0.1 s)。尤其对仲胺、叔胺、芳香族胺,甚至伯胺都有很好的选择性。目前的战略将为具有类似结构和性质的化学物质提供新的途径,特别是为打击非法药物提供新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
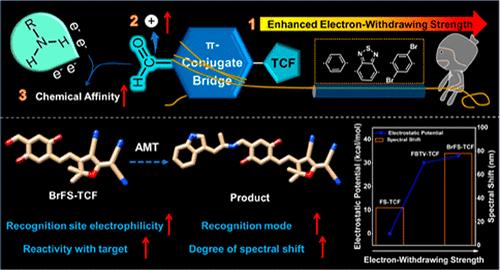
Precise Electron-Withdrawing Strength Regulation of π-Conjugate Bridge-Boosted Specific Detection toward α-Methyltryptamine
The specific fluorescent detection of α-methyltryptamine (AMT) presents a great challenge because similar amine groups and benzene rings exist in a variety of amines. Here, we show the precise modulation of the electron-withdrawing strength of the π-conjugate bridge in aldehyde-containing Schiff base-based fluorescent probes for ultratrace AMT discrimination. It is found that different electron-withdrawing groups −C6H4, −C6H2N2, and −C6H2Br2 as the π-conjugate bridge of the 2-dicyanomethylidene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran (TCF)-based probes can classify and identify organic amines with different amine nucleophilicities. Notably, the probe with −C6H2Br2 as the π-conjugate bridge, denoted as BrFS–TCF, which has the highest electrophilicity of the recognition site, shows a superior nM-level limit of detection (LOD) and an instant response time (<0.1 s) toward AMT. Especially, it shows an excellent selectivity facing the secondary amines, tertiary amines, aromatic amines, and even primary amines. The present strategy would provide a new pathway for chemical substances with similar structures and properties and especially for fighting against illegal drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


