tmt标记的定量蛋白质组学揭示了粘红酵母对干腌火腿蛋白水解的机制:结构蛋白降解、氨基酸释放和口感改善
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
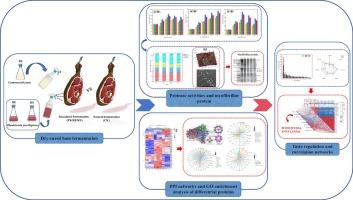
为探讨粘红酵母对金华火腿结构蛋白降解和口感发育的影响机制,研究了粘红酵母和库氏毕赤酵母对金华火腿干熟过程中蛋白水解酶活性、表面疏水性、肌原纤维微观结构、蛋白质降解、游离氨基酸和感官特性的影响。接种黏胶红霉菌EIODSF019 (RE)和黏胶红霉菌XZY63-3 (RX)的蛋白水解酶活性均高于库氏毕赤酵母XS-5 (PK)。α-螺旋的减少使肌纤维蛋白内部的疏水性基团增加,RE的表面疏水性高于PK和RX。RE蛋白水解指数最高,可能是由于RE对肌凝蛋白、肌动蛋白和肌钙蛋白的降解较多;原子力显微镜和透射电子显微镜观察到的肌原纤维的强烈破坏证实了这种变化。通过tmt标记的定量蛋白质组学分析,在RE中鉴定出36个主要来源于肌原纤维和催化相关酶的下调蛋白。肌凝蛋白、肌动蛋白和肌钙蛋白的降解对谷氨酸、赖氨酸和丙氨酸的积累反应最为强烈。偏最小二乘回归分析和相关分析显示,MYH14、MYH3、TNNI1和TNNTI的分解与鲜味、丰富度和回味的改善高度相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
TMT-labelled quantitative proteomics reveals the mechanism of Rhodotorula mucilaginosa on proteolysis of dry-cured ham: Structural protein degradation, amino acid release and taste improvement
To investigate the mechanism of Rhodotorula mucilaginosa on structural protein degradation and taste development of Jinhua ham, the effects of Rhodotorula mucilaginosa and Pichia kudriavzevii on proteolytic enzyme activities, surface hydrophobicity, myofibril microstructure, protein degradation, free amino acids and sensory attributes were investigated during the dry-ripening of Jinhua ham. The inoculation of Rhodotorula mucilaginosa EIODSF019 (RE) and Rhodotorula mucilaginosa XZY63–3 (RX) consistently exhibited higher proteolytic enzyme activities compared with Pichia kudriavzevii XS-5 (PK). The decrease of α-helix exposing more internal hydrophobic groups of myofibrillar proteins, contributed to higher surface hydrophobicity of RE compared with PK and RX. RE showed the highest proteolysis index among all groups, which could be attributed to more degradation of myosin, actin and troponin; the changes were confirmed by the intense breakdown of myofibrils observed by atomic force microscopy and transmission electron microscopy. 36 down-regulated proteins mainly derived from myofibrils and catalysis-related enzymes were identified in RE by TMT-labeled quantitative proteomics analysis. The degradation of myosin, actin and troponin showed the most intense response to the accumulation of glutamic acid, lysine and alanine. Partial least square regression analysis and correlation analysis revealed that the breakdown of MYH14, MYH3, TNNI1 and TNNTI was highly correlated with improvement of umami, richness and aftertaste.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


