基于 DACH 的手性传感平台作为可调苯甲酰胺-手性溶解剂用于 NMR 对映选择性鉴别
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
手性鉴别是一种不可缺少的工具,在确定绝对构型和确定手性化合物的对映体过量方面具有举足轻重的作用。通过简单的化学步骤首次合成了一系列对映体纯度高的反式-1,2-二氨基环己烷(trans-DACH)衍生苯并胺类化合物,并对14个变型衍生物6a-6n作为核磁共振手性溶剂化剂(csa)进行了1H核磁共振分析,用于鉴别杏仁酸(MA)的信号。通过1H, 19F和31P NMR谱扩展了CSA 6e对不同底物(包括MAs,羧酸,氨基酸衍生物和磷酸(32个例子))的高效手性识别。通过对映体分辨参数Rs来评价对映体识别的质量。对三个衍生物6c、6e和6h的单晶x射线分析有助于了解对映体识别,为有前途的核磁共振分析提供帮助。有趣的是,当CSA 6e及其立体异构体存在时,R和S构型之间的非等效质子的核磁共振信号完全相反,这可以用来建立一种直接的方法来分配不同羟基酸底物的构型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
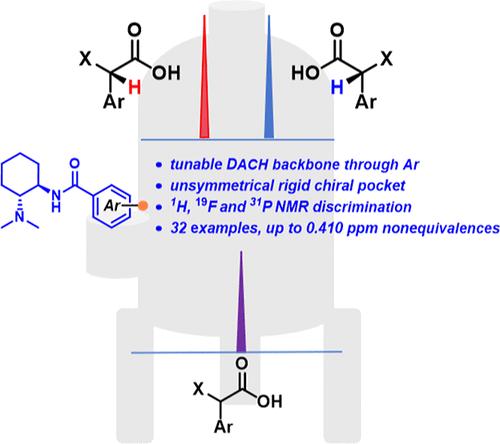
DACH-Based Chiral Sensing Platforms as Tunable Benzamide-Chiral Solvating Agents for NMR Enantioselective Discrimination
Chiral discrimination is an indispensable tool that has pivotal importance in the assignment of absolute configuration and determination of enantiomeric excess in chiral compounds. A series of enantiomerically pure trans-1,2-diaminocyclohexane (trans-DACH)-derived benzamides were first synthesized by simple chemical steps, and 14 variated derivatives 6a–6n have been assessed as NMR chiral solvating agents (CSAs) for discrimination of the signals of mandelic acid (MA) in 1H NMR analysis. The highly efficient chiral recognition of CSA 6e on different substrates, including MAs, carboxylic acids, amino acid derivatives, and phosphoric acids (32 examples), was expanded via 1H, 19F, and 31P NMR spectroscopy. The quality of enantiodiscrimination was evaluated by means of the enantioresolution parameter Rs. Single-crystal X-ray analysis of three derivatives 6c, 6e, and 6h helped to understand enantiomeric recognition for the promising NMR analysis. Interestingly, the NMR signals of nonequivalent protons between the R and S configurations were completely opposite in the presence of CSA 6e and its stereoisomer, which can be utilized to establish a straightforward method for the configuration assignment of diverse hydroxy acid substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


