更正“豆甾醇通过抑制ORP5泛素化激活mTOR信号通路促进牛乳腺上皮细胞的乳汁合成”
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
最初公布的图 7 不正确。正确数字如下。资助(编号:32202766)被错误地列入资助部分,应予以删除;更正后的资助声明如下。这些更正并不影响论文的结论。图 7.ST 通过 ORP5 促进牛奶的合成。用 ORP5-siRNA 转染 BMEC 24 小时,然后用 10 μMST 刺激 BMEC 24 小时。(B) 以β-肌动蛋白为内参,定量计算 ORP5 的蛋白条带。(C)使用 TAG 检测试剂盒分析细胞中 TAG 的含量。(D)使用 TAG 检测试剂盒分析培养液中 TAG 的含量。E)用 BODIPY 493/503(绿色)和 DAPI(蓝色)染色脂滴和细胞核。使用共聚焦显微镜观察结果(1200×,标尺 = 10 μm)。(F)使用 ImageJ 软件定量分析图 7F 中每个细胞脂滴点的面积和综合光密度(AOD)。(G、H)使用 EdU 细胞增殖检测试剂盒测定细胞增殖能力。(I)通过 Western 印迹检测 p-mTOR、mTOR、DGAT1、DGAT2、FASN、SREBP1、β-酪蛋白和细胞周期蛋白 D1 的蛋白表达水平。(J) 图 7I 中 DGAT1、DGAT2、FASN、SREBP1、β-酪蛋白和 Cyclin D1(与 β-肌动蛋白相比)以及 p-mTOR(与 mTOR 相比)的相对蛋白丰度。所有数据均以平均值 ± SEM 表示(n = 3)。与 NC 组相比,**p < 0.01。本研究由吉林省自然科学基金资助(项目编号:20220101302JC)。本文尚未被其他出版物引用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
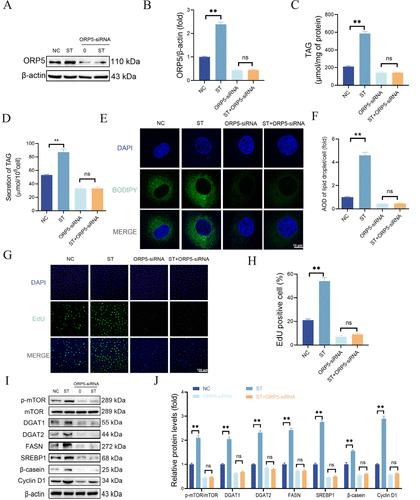
Correction to “Stigmasterol Activates the mTOR Signaling Pathway by Inhibiting ORP5 Ubiquitination to Promote Milk Synthesis in Bovine Mammary Epithelial Cells”
Figure 7 as originally published was incorrect. The correct figures are given below. Funding (No. 32202766) was included incorrectly in the funding section and should be removed; the corrected Funding statement is below. The corrections do not affect the conclusions of the paper. Figure 7. ST promotes the synthesis of milk through ORP5. BMECs were transfected with ORP5-siRNA for 24 h, followed by stimulation with 10 μMST for 24 h. (A) The protein expression levels of ORP5 were detected by Western blotting. (B) The protein bands of ORP5 were quantitatively calculated with β-actin as the internal reference. (C) The content of TAG in cells was analyzed using a TAG detection kit. (D) The content of TAG in the culture medium was analyzed using a TAG detection kit.(E) Lipid droplets and nuclei were stained with BODIPY 493/503 (green) and DAPI (blue). Results were visualized using a confocal microscope (1200×, scale bar = 10 μm). (F) The area and integrated optical density (AOD) of lipid droplet spots per cell in Figure 7F were quantitatively analyzed using ImageJ software. (G, H) The cell proliferation capacity was determined using the EdU cell proliferation assay kit. (I) The protein expression levels of p-mTOR, mTOR, DGAT1, DGAT2, FASN, SREBP1, β-casein, and Cyclin D1 were detected by Western blotting. (J) The relative protein abundance of DGAT1, DGAT2, FASN, SREBP1, β-casein, and Cyclin D1 (compared to β-actin) as well as p-mTOR (compared to mTOR) from Figure 7I. All data are presented as mean ± SEM (n = 3). **p < 0.01 versus the NC group. This study was supported by the Natural Science Foundation of Jilin Province (project no. 20220101302JC). This article has not yet been cited by other publications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


