体外分离快速反应高亲和性抗原,用于连续无残留检测凝血酶原
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
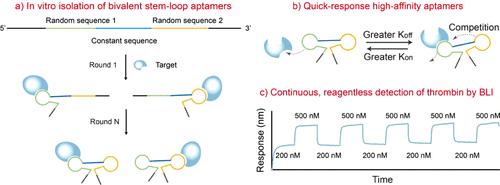
In Vitro Isolation of Quick-Response High-Affinity Aptamers for Continuous and Reagentless Detection of Thrombin
Continuous and reagentless biomolecular detection technologies are bringing an evolutionary influence on disease diagnostics and treatment. Aptamers are attractive as specific recognition probes because they are capable of regeneration without washing. Unfortunately, the affinity and dissociation kinetics of the aptamers developed to date show an inverse relationship, preventing continuous and reagentless detection of protein targets due to their low dissociation rates. Here, we describe an in vitro aptamer isolation strategy that enriches quick-response, high-affinity bivalent protein-binding aptamers. The method is general, as evidenced by the isolation of aptamers targeting thrombin and human serum albumin. We then demonstrated the excellent regeneration capability of the isolated thrombin aptamers using biolayer interferometry. The sensors instantly responded to alternating concentration changes of thrombin at nanomolar levels (200–500 nM), reaching highly consistent equilibrium signals within 10 s. In contrast, the well-known thrombin-binding aptamers, TBA-15 and TBA-29, were not capable of regeneration. Our study provides a simple means to obtain quick-response, high-affinity protein-binding aptamers. It can also be used for the isolation of aptamer pairs, which has been demonstrated to be quite challenging. Our study also provides insights into the rational design of aptamers to control their binding thermodynamics and kinetics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


