基于肽组学和分子模拟的鳞片防冻肽特异性筛选及其作用机理研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究旨在探讨食源性水解肽的冷冻保护机制,开发新型冷冻保护剂,以提高冷冻食品的质量。采用酶解法制备了日本鱼鳞防冻肽(ejafp),其对鱼的保护效果是传统防冻剂的4倍。此外,Ej-AFP能将63.60%的冰晶控制在600 μm2以下。利用冰亲和技术和肽组学技术纯化了三个抗冻肽序列。与j- afp相比,这些序列的抗冻活性提高了21.75%,热滞活性提高了1°C。分子模拟表明,冰结合表面通过氢键与冰晶相互作用,而非冰结合表面破坏了水分子的有序排列。这导致在肽周围形成结构紧密的水合层,增加冰晶表面的曲率,从而在控制冰晶生长方面显示出显著的防冻活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
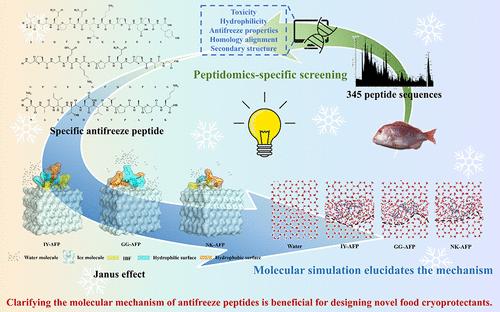
Peptidomics & Molecular Simulation-Based Specific Screening of Antifreeze Peptides from Evynnis japonica Scale and the Action Mechanism
This study aims to explore the cryoprotective mechanisms of food-derived hydrolyzed peptides and develop novel cryoprotectants to enhance the quality of frozen foods. Evynnis japonica scale antifreeze peptides (Ej-AFP) were prepared using enzymatic hydrolysis, which had a 4-fold increase in protection efficiency for surimi compared to traditional cryoprotectants. Furthermore, Ej-AFP was able to control 63.60% of the ice crystals to sizes below 600 μm2. Three antifreeze peptide sequences were purified by using ice-affinity techniques and peptidomics. These sequences demonstrated a 21.75% enhancement in antifreeze activity and an increase of 1 °C in thermal hysteresis activity compared to Ej-AFP. Molecular simulation-elucidated ice-binding surface interacts with ice crystals through hydrogen bonds, while the nonice-binding surface disrupts the orderly arrangement of water molecules. This results in a tightly structured hydration layer around the peptide, increasing the curvature of the ice crystal surface and thereby demonstrating significant antifreeze activity in controlling ice crystal growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


