单细胞外囊泡纳米化通用协议 (SEVEN-UP):用于定量表征单个细胞外囊泡的可访问成像平台
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
细胞外囊泡(EVs)是一种从所有细胞中脱落的膜包裹纳米颗粒,与细胞的关键功能密切相关。此外,电动汽车最近成为令人兴奋的治疗方式、传递载体和生物标志物来源。然而,电动汽车很难表征,因为它们通常很小,在尺寸、来源和分子含量上都是异质的。单EV方法的最新进展通过提供评估单个囊泡的敏感工具解决了其中的一些挑战;其中一个例子是我们最近开发的单细胞外囊泡纳米显微镜(SEVEN)方法。然而,由于这些工具需要高度专业化的设备,因此一般研究界通常无法普遍使用这些工具。在这里,我们展示了如何通过一种采用超分辨率径向波动(SRRF)显微镜和先进数据分析的新方法使单个EV研究大众化。SRRF与各种显微镜和荧光团兼容。本文通过结合亲和分离(基于SEVEN的分析方案)、SRRF显微镜和基于机器学习的EV评估支持的新分析算法来量化单个EV。使用SEVEN,我们首先优化了工作流程,并验证了在宽视场和全内反射荧光显微镜上获得的数据。我们进一步证明,我们的方法,我们称之为七通用协议(SEVEN-UP),可以可靠地评估血浆和重组ev的数量、大小和含量。最后,我们使用该平台评估条件细胞培养基中ev中的RNA。使用SYTO RNASelect染料,我们发现来自HEK 293T细胞的EVs中有18%含有RNA;与普通电动汽车相比,这些电动汽车明显更大。总之,我们为研究界开发了一种经济的、多参数的、单一EV表征方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
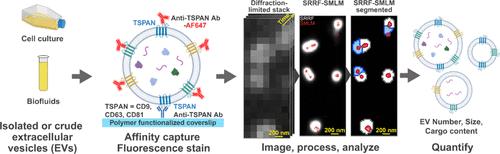
Single Extracellular VEsicle Nanoscopy-Universal Protocol (SEVEN-UP): Accessible Imaging Platform for Quantitative Characterization of Single Extracellular Vesicles
Extracellular vesicles (EVs), membrane-encapsulated nanoparticles shed from all cells, are tightly involved in critical cellular functions. Moreover, EVs have recently emerged as exciting therapeutic modalities, delivery vectors, and biomarker sources. However, EVs are difficult to characterize, because they are typically small and heterogeneous in size, origin, and molecular content. Recent advances in single EV methods have addressed some of these challenges by providing sensitive tools for assessing individual vesicles; one example is our recently developed Single Extracellular VEsicle Nanoscopy (SEVEN) approach. However, these tools are typically not universally available to the general research community, as they require highly specialized equipment. Here, we show how single EV studies may be democratized via a novel method that employs super-resolution radial fluctuations (SRRF) microscopy and advanced data analysis. SRRF is compatible with a wide range of microscopes and fluorophores. We herein quantified individual EVs by combining affinity isolation (analytical protocol based on SEVEN) with SRRF microscopy and new analysis algorithms supported by machine learning-based EV assessment. Using SEVEN, we first optimized the workflow and validated the data obtained on wide-field and total internal reflection fluorescence microscopes. We further demonstrated that our approach, which we call the SEVEN-Universal Protocol (SEVEN-UP), can robustly assess the number, size, and content of plasma and recombinant EVs. Finally, we used the platform to assess RNA in EVs from conditioned cell culture media. Using SYTO RNASelect dye, we found that 18% of EVs from HEK 293T cells appear to contain RNA; these EVs were significantly larger compared with the general EV population. Altogether, we developed an economical, multiparametric, single EV characterization approach for the research community.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


