用二维红外光谱测量较长时间尺度上蛋白质动力学的振动标记——氰基硒代半胱氨酸的快速生成
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
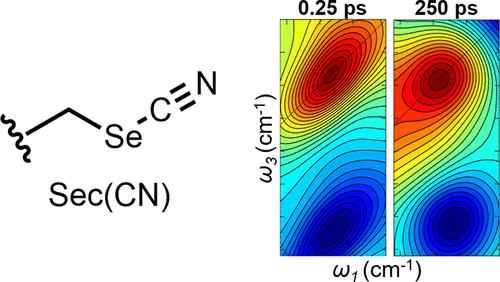
二维红外光谱是测量分子非均质性和动力学的一种强大的技术,具有很高的时空分辨率。该方法可以应用于通过结合频率分辨振动标签来表征蛋白质的特定残基。然而,由于二维红外吸收带的衰减,振动标签的激发态寿命限制了二维红外光谱可以测量的动力学时间尺度。为了延长这个时间尺度,需要寻找寿命更长的振动标签。抑制分子内能量松弛的有效方法是通过插入重原子桥将振动与分子的其余部分隔离开来。虽然这一策略已经通过功能化氨基酸的产生得到了证明,但它们选择性结合到蛋白质中的直接途径往往是不清楚的。在半胱氨酸上连接氰基生成硫氰酸盐的简单方法使其被用作蛋白质的振动标记。我们证明了一个类似的途径可以用于引入氰基硒代半胱氨酸来生成含有更重桥原子的硒氰酸酯振动标签。我们通过红外泵浦探针和二维红外光谱证实,根据溶剂的不同,更长的振动寿命为100-250 ps,这使得二维红外光谱的收集能够在更长的时间尺度上测量频率动态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Facile Generation of Cyanoselenocysteine as a Vibrational Label for Measuring Protein Dynamics on Longer Time Scales by 2D IR Spectroscopy
Two-dimensional infrared (2D IR) spectroscopy is a powerful technique for measuring molecular heterogeneity and dynamics with a high spatiotemporal resolution. The methods can be applied to characterize specific residues of proteins by incorporating frequency-resolved vibrational labels. However, the time scale of dynamics that 2D IR spectroscopy can measure is limited by the vibrational label’s excited-state lifetime due to the decay of 2D IR absorption bands. To extend this time scale, vibrational labels with longer lifetimes are sought. An effective approach to inhibiting intramolecular energy relaxation is to isolate the vibration from the rest of the molecule by inserting a heavy atom bridge. Although this strategy has been demonstrated through the generation of functionalized amino acids, a straightforward route to their selective incorporation into proteins is often unclear. A facile approach for the attachment of a cyano group at cysteine to generate a thiocyanate has contributed to its adoption as a vibrational label of proteins. We demonstrate that an analogous route can be used for introducing cyanoselenocysteine to generate a selenocyanate vibrational label containing a heavier bridge atom. We confirm by infrared pump–probe and 2D IR spectroscopy longer vibrational lifetimes of 100–250 ps, depending on the solvent, which enable the collection of 2D IR spectra to measure frequency dynamics on longer time scales.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


