天然植物产物结构优化:新型2-异丙醇-4-甲氧基-7-烷基/芳基羰基-(E)-乙烯基-2,3-二氢苯并呋喃的构建、杀虫活性和毒理学研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
近年来,天然生物活性产物的结构优化已成为发现新型候选农药的重要途径之一。以蛇床子为先导化合物,合成了一系列新的2-异丙醇-4-甲氧基-7-烷基/芳基羰基-(E)-乙烯基-2,3-二氢苯并呋喃衍生物。化合物3、4、6、9、11、29和31的立体构型通过x射线单晶学得到了证实。研究了以蛇床子为原料,通过环氧化和重排反应制备2-异丙醇-2,3-二氢苯并呋喃的有效方法。化合物31 (R = CH2CH2Ph;LC50为0.759 mg/mL),杀虫活性是蛇床子素的1.9倍;抗朱砂叶螨,化合物34 (R = (CH2)9CH3;LC50: 0.401 mg/mL)的杀螨活性是蛇床子的3.3倍,防治效果较好。通过扫描电镜(SEM)成像方法,证实化合物34的杀螨活性可能与破坏朱砂皮角质层嵴有关。化合物34可作为潜在的杀螨剂进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
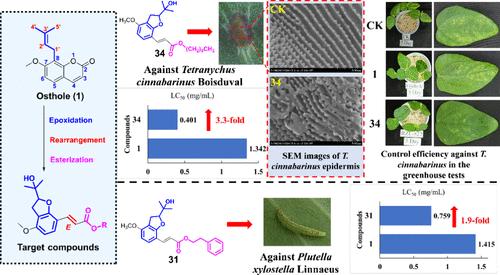
Structural Optimization of Natural Plant Products: Construction, Pesticidal Activities, and Toxicology Study of New 2-Isopropanol-4-methoxy-7-alkyl/aryloxycarbonyl-(E)-vinyl-2,3-dihydrobenzofurans
Recently, the structural optimization of natural bioactive products has been one of the important ways to discover new pesticide candidates. Based on osthole as a lead compound, herein, a series of new 2-isopropanol-4-methoxy-7-alkyl/aryloxycarbonyl-(E)-vinyl-2,3-dihydrobenzofuran derivatives were synthesized. Steric configurations of compounds 3, 4, 6, 9, 11, 29, and 31 were confirmed by X-ray monocrystallography. Notably, an efficient method for preparation of 2-isopropanol-2,3-dihydrobenzofurans from osthole by the epoxidation and rearrangement reactions was developed. Against Plutella xylostella Linnaeus, compound 31 (R = CH2CH2Ph; LC50: 0.759 mg/mL) displayed a 1.9-fold insecticidal activity compared to that of osthole; against Tetranychus cinnabarinus Boisduval, compound 34 (R = (CH2)9CH3; LC50: 0.401 mg/mL) exhibited a 3.3-fold acaricidal activity and good control effects compared to those of osthole. By the scanning electron microscope (SEM) imaging method, it was demonstrated that the acaricidal activity of compound 34 may be related to the damage of the cuticle layer crest of T. cinnabarinus. Compound 34 could be further studied as a potential acaricide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


