提高活性和稳定性的5-氨基乙酰酸合酶的合理设计工程
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
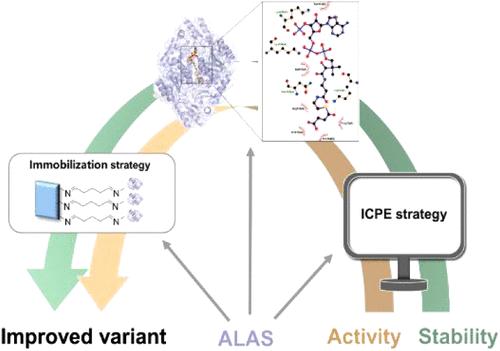
5-氨基乙酰丙酸合成酶(ALAS)是合成重要生物合成中间体5-氨基乙酰丙酸(ALA)的关键限速酶。但其活性低,稳定性差,影响了催化效率。通过等温压缩率(βT)微扰工程和两种热稳定性预测算法,我们获得了突变体I325M/V390Y/H391I (T6),其比活性(2.53 U/mg)比野生型提高了7.0倍。此外,分子动力学模拟表明,分子间相互作用、底物通道和结合袋的积极变化是T6催化活性提高的原因。此外,将T6固定在磁性壳聚糖纳米颗粒上,经过10个反应循环后,其活性仍保持在原来的73.5%。综上所述,本研究通过组合方法构建了一个优良的ALA变体,为ALA的合成提供了一个新的概念,并为相关领域的工业酶优化提供了有价值的基准。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rational Design Engineering of 5-Aminolevulinate Synthase with Activity and Stability Enhancement
5-Aminolevulinic acid synthase (ALAS) is the key rate-limiting enzyme in the synthesis of the vital biosynthetic intermediate 5-aminolevulinic acid (ALA). However, its catalytic efficiency is compromised due to its low activity and poor stability. Here, we obtained the mutant I325M/V390Y/H391I (T6), which exhibited a 7.0-fold increase in specific activity (2.53 U/mg) compared to the wild type through the application of isothermal compressibility (βT) perturbation engineering in conjunction with two thermal stability prediction algorithms. Moreover, molecular dynamics simulations indicate that positive changes in intermolecular interactions, the substrate channel, and the binding pocket account for the improved catalytic activity of T6. Furthermore, T6 was immobilized on magnetic chitosan nanoparticles, maintaining 73.5% of its original activity after 10 reaction cycles. Overall, combination approaches were employed to construct a superior ALAS variant, providing a novel concept for the synthesis of ALA and a valuable benchmark for optimizing industrial enzymes in related fields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


