克劳氏碱杆菌的尿酸酶——降低豆制品尿酸含量的工业应用候选物
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
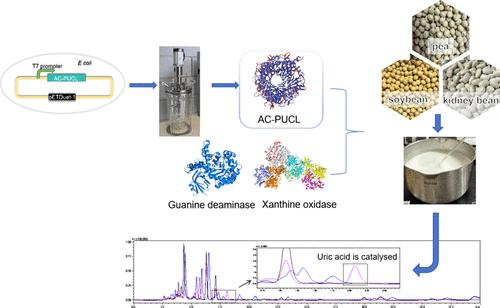
微生物尿酸酶是嘌呤降解和低嘌呤食品生产的重要酶。对于低嘌呤食物,必须建立高酶活性和适当的最佳pH。粗神经孢子菌、彩色链霉菌、克劳氏碱杆菌、枯草芽孢杆菌和干酪短杆菌的尿酸酶在大肠杆菌中异种表达。clusii碱性杆菌尿酸酶(AC-PUCL)在37℃、pH 7.0条件下活性最高,为249.19 IU/mL。这接近植物性食物的pH值。AC-PUCL的Km和Km/Kcat值分别为30.12 μM和1.46 s-1 μM - 1。此外,不同来源的尿酸酶的晶体结构表明,氢键可以增强底物亲和力和较强的酶活性。此外,适当大小的活性口袋可能有助于提高酶的活性。最后,AC-PUCL可降低大豆、豌豆和芸豆中的嘌呤物质,且在pH 8.6时无法检测到残留的尿酸,具有很好的工业应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Uricase from Alkalihalobacillus clausii, a Candidate for Industrial Application of Reducing Uric Acid Content of Bean Products
Microbial uricase is an essential enzyme in purine degradation and the development of low-purine food. High enzyme activity and an appropriate optimum pH must be established for low-purine food. Uricases from Neurospora crassa, Streptomyces coelicolor, Alkalihalobacillus clausii, Bacillus subtilis, and Brevibacterium casei were heterologously expressed in Escherichia coli. Uricase from Alkalihalobacillus clausii (AC-PUCL) showed the most potent enzyme activity (249.19 IU/mL) at 37 °C and pH 7.0. This is close to the pH of plant-based food. The Km and Km/Kcat values of AC-PUCL were 30.12 μM and 1.46 s–1 μM–1, respectively. Furthermore, the crystal structures of uricases from different sources revealed that hydrogen bonds could enhance substrate affinity and strong enzyme activity. In addition, the high enzyme activity may be contributed by the active pockets with an appropriate size. Finally, AC-PUCL helped reduce the purine substances in soybean, pea, and kidney bean, with residual uric acid can not be detected at pH 8.6, suggesting a promising industrial application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


