毛细管等电聚焦蛋白质和多肽的在线cIEF-ESI界面与改进的质谱特性
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
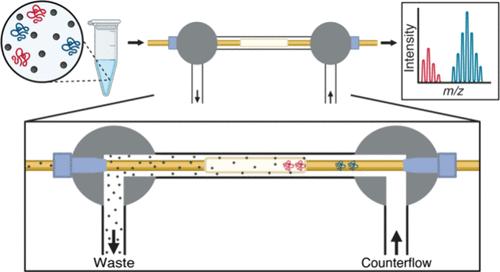
使用质谱(MS)进行完整蛋白分析是一项重要的技术,可以表征和提供蛋白质复杂性的全面概述。这也是蛋白质组学中“自上而下”方法描述单个蛋白质翻译后修饰(PTMs)的蛋白质形态的基础。基于质谱的完整蛋白质分析受益于电喷雾电离之前的高分辨率分离。毛细管等电聚焦(cIEF)是一种高分辨率的蛋白质和多肽分离技术,能够分离蛋白质形态。结合cIEF的MS检测可以在分子水平上分离、检测和表征蛋白质形态。然而,带MS检测的cIEF是一个折衷的过程。cIEF所需的两性电解质浓度与质谱仪污染是相互排斥的。我们改进了在线cIEF- esi - ms界面,在cIEF后和电喷雾电离之前在线还原(脱盐)氨基酸两性电解质。在原理验证实验中,观察到有界面的电泳曲线下面积比没有界面的面积增加了90%。蛋白标准品包括细胞色素C、肌红蛋白和α-酪蛋白的蛋白形态进行分离和分离,具有高重复性。该界面不影响cIEF pH梯度分离的线性度,实现了高线性度,R2为0.99。此外,BSA的胰蛋白酶消化显示肽的基线分辨率只有0.02 pI单位差,半最大值时的全宽度平均为7.1 s。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Capillary Isoelectric Focusing of Proteins and Peptides Using an In-Line cIEF-ESI Interface with Improved MS Characteristics
Intact protein analysis using mass spectrometry (MS) is an important technique to characterize and provide a comprehensive overview of protein complexity. It is also the basis of “top-down” approaches in proteomics to describe the proteoforms of single protein’s post-translational modifications (PTMs). MS-based analysis of intact proteins benefits from high-resolution separations prior to electrospray ionization. Capillary isoelectric focusing (cIEF) is a high-resolution separation for proteins and peptides which is capable of separating proteoforms. MS detection coupled to cIEF can separate, detect, and characterize proteoforms at the molecular level. However, cIEF with MS detection is a compromised process. The concentration of ampholytes required for cIEF is mutually exclusive with mass spectrometer contamination. We have improved an online cIEF-ESI-MS interface to reduce (desalt) amino acid ampholytes in-line after cIEF and prior to electrospray ionization. In proof of principle experiments, >90% increase in area under the curve of the electropherograms was observed with the interface compared to without the interface. Protein standards including proteoforms of cytochrome C, myoglobin, and α-casein were separated and resolved with high reproducibility. The interface did not compromise the linearity of the cIEF pH gradient separations, achieving a high linearity with a R2 of 0.99. In addition, a tryptic digest of BSA demonstrates baseline resolution of peptides with as little as 0.02 pI unit difference and a full width at half-maximum average of 7.1 s.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


