神经退行性疾病在Simoa平台上的超灵敏蛋白聚集定量分析
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
纳米级聚集体在神经退行性疾病如阿尔茨海默病和帕金森病的发病机制中发挥关键作用。然而,对复杂生物样品(如生物体液和死后脑组织)中的这些聚集体进行量化一直具有挑战性,因为它们的浓度低且体积小,因此需要开发具有高灵敏度和特异性的方法。在这里,我们利用Quanterix Simoa平台开发了超灵敏的检测方法来检测α-synuclein, β-淀粉样蛋白和tau聚集体,包括那些常见的翻译后修饰,如α-synuclein的截断和tau聚集体的AT8磷酸化。所有测定法在低pM范围内均有检出限。作为这项工作的一部分,我们开发了二氧化硅纳米颗粒校准器,允许对所有聚集体进行量化。通过变性和交叉反应性实验验证了这些检测方法的聚集体和靶标特异性。然后,我们将这些分析应用于阿尔茨海默病的大脑匀浆样本和对照样本,证明了它们对死后组织的适用性。最后,我们通过检测早期阿尔茨海默病患者血清样本中的聚集物,探索了这些检测方法在基于血液的诊断中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
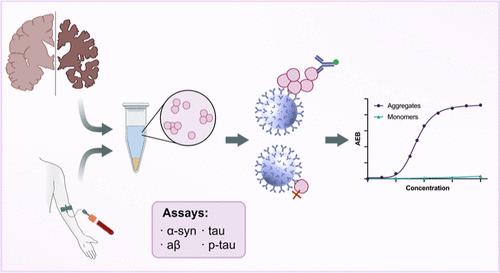
Ultrasensitive Protein Aggregate Quantification Assays for Neurodegenerative Diseases on the Simoa Platform
Nanoscale aggregates play a key role in the pathogenesis of neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. However, quantifying these aggregates in complex biological samples, such as biofluids and postmortem brain tissue, has been challenging due to their low concentration and small size, necessitating the development of methods with high sensitivity and specificity. Here, we have developed ultrasensitive assays utilizing the Quanterix Simoa platform to detect α-synuclein, β-amyloid and tau aggregates, including those with common posttranslational modifications such as truncation of α-synuclein and AT8 phosphorylation of tau aggregates. All assays had a detection limit in the low pM range. As a part of this work, we developed silica-nanoparticle calibrators, allowing for the quantification of all aggregates. These assays were validated for aggregate and target specificity through denaturation and cross-reactivity experiments. We then applied these assays to brain homogenate samples from Alzheimer’s disease and control samples, demonstrating their applicability to postmortem tissue. Lastly, we explored the potential of these assays for blood-based diagnostics by detecting aggregates in serum samples from early Alzheimer’s disease patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


