重组人α-葡萄糖苷酶在HEK293细胞中的表达
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
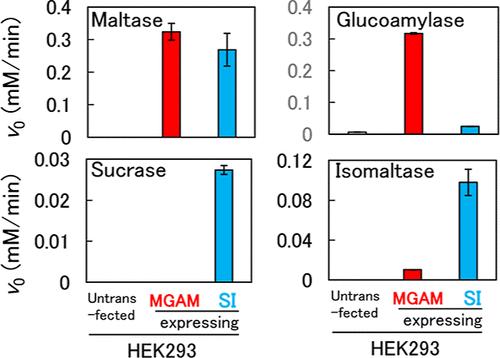
在哺乳动物中,肠道α-葡萄糖苷酶以麦芽糖酶-葡萄糖淀粉酶复合物(MGAM)和蔗糖-异麦芽糖酶复合物(SI)的形式存在。在这项研究中,我们在人胚胎肾293 (HEK293)细胞中短暂表达了人MGAM和SI。在pH 6.0和37°C下,表达mgam的HEK293细胞提取物(MGE)表现出麦芽糖酶、葡萄糖淀粉酶和异麦芽糖酶的活性,但不表现出蔗糖酶的活性,而表达si的HEK293细胞提取物(SIE)表现出蔗糖酶、异麦芽糖酶和麦芽糖酶的活性,但不表现出葡萄糖淀粉酶的活性。麦芽糖水解MGE的表观Km值是麦芽糖、蔗糖和异麦芽糖水解SIE的14-26%。蔗糖和异麦芽糖水解MGE和SIE的表观Vmax分别为麦芽糖水解的0%和6%和10%和42%。这些结果表明,MGAM和SI的麦芽糖酶活性高于蔗糖酶和异麦芽糖酶。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Expression of Recombinant Human α-Glucosidase in HEK293 Cells
In mammals, intestinal α-glucosidase exists as a maltase–glucoamylase complex (MGAM) and a sucrase–isomaltase complex (SI). In this study, we transiently expressed human MGAM and SI in human embryonic kidney 293 (HEK293) cells. At pH 6.0 and 37 °C, the MGAM-expressing HEK293 cells extract (MGE) exhibited maltase, glucoamylase, and isomaltase activities but not sucrase activity, whereas the SI-expressing HEK293 cells extract (SIE) exhibited sucrase, isomaltase, and maltase activities but not glucoamylase activity. The apparent Km value of the MGE for maltose hydrolysis was 14–26% of that of the SIE for maltose, sucrose, and isomaltose hydrolysis. The respective apparent Vmax values of the MGE and SIE for sucrose and isomaltose hydrolysis were 0% and 6% and 10% and 42% of those for maltose hydrolysis. These results indicated that the maltase activities of MGAM and SI were higher than those of sucrase and isomaltase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


