新型吡唑-羧酰胺类转酮醇酶抑制剂的设计、合成及除草活性研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
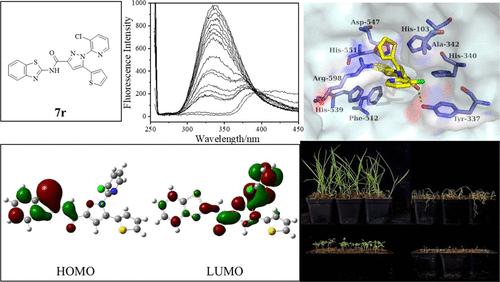
转酮醇酶(设备;EC 2.2.1.1)已被确定为潜在的新除草剂靶标。为了发现靶向TKL的高效除草活性化合物,提高其先导化合物的结构多样性,通过对含吡唑苯氧酰胺化合物4u的结构优化,设计合成了一系列吡唑-羧酰胺7a-7v。在所合成的化合物中,化合物7r在温室中对马地黄(Digitaria sanguinalis, Ds)和红苋菜(Amaranthus retroflexus, Ar)的小杯法(抑制率约95%,100 mg/L)和叶面喷雾法(抑制率90%以上,150 gai /ha)均具有优异的除草效果,优于阳性对照硝磺隆。更显著的是,即使在375 g ai/ hm2时,化合物7r对玉米和小麦也表现出良好的作物选择性。高除草活性化合物的作用模式(MOA)研究,包括酶抑制活性、荧光猝灭实验以及与配体的分子对接分析,表明化合物7r是典型的TKL抑制剂,而苯并噻唑环是SvTKL抑制活性的重要基序。综上所述,化合物7r可作为新型MOA除草剂开发的潜在候选物,用于玉米和小麦的田间杂草防治。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, Synthesis, and Herbicidal Activity Study of Novel Pyrazole-Carboxamides as Potential Transketolase Inhibitors
Transketolase (TKL; EC 2.2.1.1) has been identified as a potential new herbicide target. In order to discover highly herbicidal active compounds targeting TKL and improve their structural diversity for lead compounds, a series of pyrazole-carboxamides 7a–7v were designed and synthesized through structural optimization for pyrazole-containing phenoxy amide compound 4u. Among the synthesized compounds, compound 7r possessed excellent herbicidal efficacy against Digitaria sanguinalis (Ds) and Amaranthus retroflexus (Ar) by the small cup method (the inhibition about 95%, 100 mg/L) and the foliar spray method (the inhibition over 90%, 150 g ai/ha) in a greenhouse, which were superior to that of the positive control nicosulfuron. More significantly, compound 7r displayed good crop selectivity toward both maize and wheat even at 375 g of ai/ha. The studies on mode of action (MOA) of high herbicidal active compounds, including the enzyme inhibition activity, fluorescent quenching experiments, and molecular docking analysis between Setaria viridis (Sv)TKL and ligand, suggested that compound 7r acts as a typical TKL inhibitor, and the benzothiazole ring is an important motif for SvTKL inhibition activity. Above all, compound 7r could be a potential candidate for the development of herbicides with new MOA for weed control in maize and wheat field.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


