日本晚疫病中的一种热稳定性过敏原--托品肌苷的鉴定和过敏性分析日本晚疫霉的一种热稳定过敏原
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
一种普遍存在的水生美味,已知在某些个体中会引起过敏反应。然而,对其致敏成分的调查仍然明显不足。本研究通过LC-MS /MS验证了一种约35 kDa的热稳定蛋白为原肌球蛋白(tropomyosin, TM)。通过PCR扩增得到全长852 bp的开放阅读框,编码284个氨基酸。80例鱼致敏患者中,TM在大肠杆菌中表达的ige结合频率为22.5%。此外,TM在7例患者中具有激活嗜碱性细胞的能力。在Balb/c小鼠模型中,与PBS组相比,TM组特异性抗体(IgE、IgG1、IgG2a)、CD19+ B细胞、IL-4、IL-10水平显著升高。然而,CD4+ TCR-β、CD4+ CD25+ foxp3 +细胞和IFN-γ的情况恰恰相反。这些关于过敏原的发现有助于对鱼类过敏进行成分解析诊断和治疗研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
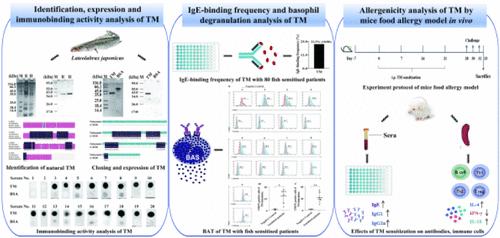
Identification and Allergenicity Analysis of Tropomyosin: A Heat-Stable Allergen in Lateolabrax japonicus
Lateolabrax japonicus, a prevalent aquatic delicacy, is known to elicit allergic reactions in certain individuals. Nevertheless, the investigation into its allergenic components has remained notably inadequate. In the research, an approximately 35 kDa heat-stable protein of L. japonicus raw/steamed extracts was verified as tropomyosin (TM) by LC–MS/MS. Open reading frame of TM (852 bp) was acquired via PCR amplification, encoding 284 amino acids. The IgE-binding frequency of TM expressed in Escherichia coli was 22.5% among 80 fish-sensitized patients. Furthermore, TM had the ability to activate basophils in 7 patients. In the Balb/c mice model, compared with the PBS group, the levels of specific antibodies (IgE, IgG1, and IgG2a), CD19+ B cells, IL-4, and IL-10 were significantly increased in the TM group. However, the opposite was indeed the case for CD4+ TCR-β, CD4+ CD25+ Fox p 3+ cells, and IFN-γ. These findings regarding an allergen assist in conducting component-resolved diagnoses and therapeutic research for fish allergy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


