揭示 Fusarium oxysporum (Fo32931 和 FocII5) 对杀真菌剂 Phenamacril 的不同敏感性:从计算和实验角度看
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
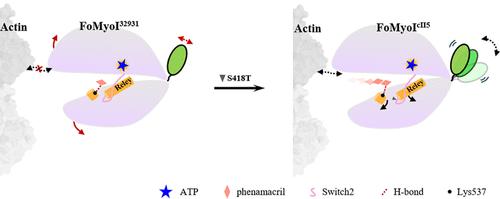
Fo32931 和 FoCII5 是致病性丝状真菌 Fusarium oxysporum(Fo)的两个亚型。Phenamacril(PHA)是一种针对肌球蛋白 I 的镰刀菌特异性杀菌剂,尽管肌球蛋白 I 的 PHA 结合袋中只有两个氨基酸不同,但它在 Fo32931 中表现出明显的菌丝生长抑制作用,而在 FocII5 中却表现出微弱的抗性。在本研究中,我们旨在通过计算方法和生化验证,阐明草孢霉肌球蛋白 I 变体(FoMyoI32931 和 FoMyoIcII5)对 phenamacril 不同敏感性的分子基础。结果表明,苯那普利可作为 FoMyoI32931 的异构抑制剂,抑制转换杠杆结构域(CLD)在与 ATP 结合时的大幅振荡,并促进外部裂隙的打开,从而进一步损害蛋白质的功能。在 FoMyoI32931 中,PHA 明显降低了 CLD(尤其是转换器)与催化中心之间的耦合,减弱了 CLD 对马达结构域的响应。通过残基突变实验,我们发现 FoMyoIcII5 中的 S418T 取代是导致 FocII5 苯马普利敏感性降低的关键。根据微尺度热泳(MST)检测和口袋构象分析,S418T突变扰乱了口袋残基,尤其是Lys537的取向,导致口袋变松,Lys537与苯马普利的相互作用减弱,从而降低了FoMyoIcII5与苯马普利的结合亲和力。这些发现从分子和结构两个角度深入揭示了 FoCII5 对 phenamacril 的敏感性降低的原因,并将指导设计针对 FoCII5 等抗性镰刀菌属的新型抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Uncovering the Differed Susceptibility of Fusarium oxysporum (Fo32931 and FocII5) to Fungicide Phenamacril: From Computational and Experimental Perspectives
Fo32931 and FoCII5 are two subtypes of Fusarium oxysporum (Fo), a pathogenic filamentous fungus. Phenamacril (PHA), a Fusarium-specific fungicide that targets myosin I, exhibits significant hyphal growth inhibition in Fo32931 but shows weak resistance in FocII5, despite only two amino acid differences in the PHA-binding pocket of myosin I. In this study, we aim to elucidate the molecular basis for the differential sensitivity ofF. oxysporum myosin I variants (FoMyoI32931 and FoMyoIcII5) to phenamacril through computational methods and biochemical validation. The results suggest that phenamacril functions as an allosteric inhibitor for FoMyoI32931, inhibiting the large oscillation of the converter lever domain (CLD) upon ATP binding and promoting the opening of the outer cleft, further impairing protein function. PHA significantly reduced the coupling between the CLD, especially the converter, and the catalytic center, diminishing the response of the CLD to the motor domain in FoMyoI32931. From the residue mutation experiment, we found that the S418T substitution in FoMyoIcII5 is the key to the reduced phenamacril sensitivity of FocII5. According to the microscale thermophoresis (MST) assay and pocket conformation analysis, the S418T mutation disturbs the orientation of pocket residues, especially Lys537, leading to a looser pocket and reduced interaction between Lys537 and phenamacril, which lowers the binding affinity of FoMyoIcII5 for phenamacril. These findings provide deeper insights into the reasons for the lower sensitivity of FoCII5 to phenamacril from both molecular and structural perspectives and will also guide the design of novel inhibitors against resistant Fusarium spp., like FoCII5.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


